[English] 日本語
 Yorodumi
Yorodumi- EMDB-40655: Phosphoinositide phosphate 3 kinase gamma bound with ADP and two ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Phosphoinositide phosphate 3 kinase gamma bound with ADP and two Gbetagamma subunits in State 2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Phosphoinositide 3-Kinase / Phosphoinositide 3-Kinase /  Chemotaxis / Chemotaxis /  Cancer / Cancer /  SIGNALING PROTEIN SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationOlfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Activation of the phototransduction cascade / 1-phosphatidylinositol-3-kinase regulator activity / phosphatidylinositol 3-kinase complex, class IB / phosphatidylinositol-mediated signaling / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma ...Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Activation of the phototransduction cascade / 1-phosphatidylinositol-3-kinase regulator activity / phosphatidylinositol 3-kinase complex, class IB / phosphatidylinositol-mediated signaling / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / G alpha (12/13) signalling events / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Thrombin signalling through proteinase activated receptors (PARs) / Ca2+ pathway / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events /  phosphatidylinositol-4,5-bisphosphate 3-kinase / G alpha (q) signalling events / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / phosphatidylinositol phosphate biosynthetic process / photoreceptor disc membrane / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / sensory perception of taste / G-protein beta-subunit binding / phosphatidylinositol-4,5-bisphosphate 3-kinase / G alpha (q) signalling events / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / phosphatidylinositol phosphate biosynthetic process / photoreceptor disc membrane / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / sensory perception of taste / G-protein beta-subunit binding /  heterotrimeric G-protein complex / signaling receptor complex adaptor activity / retina development in camera-type eye / heterotrimeric G-protein complex / signaling receptor complex adaptor activity / retina development in camera-type eye /  GTPase binding / phospholipase C-activating G protein-coupled receptor signaling pathway / GTPase binding / phospholipase C-activating G protein-coupled receptor signaling pathway /  kinase activity / cell population proliferation / G protein-coupled receptor signaling pathway / kinase activity / cell population proliferation / G protein-coupled receptor signaling pathway /  phosphorylation / phosphorylation /  GTPase activity / protein-containing complex binding / GTPase activity / protein-containing complex binding /  membrane / membrane /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Sus scrofa (pig) / Sus scrofa (pig) /   Bos taurus (cattle) Bos taurus (cattle) | |||||||||
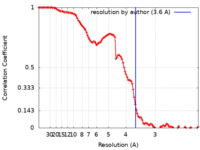
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Chen C-L / Tesmer JJG / Bandekar SJ / Cash J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Molecular basis for Gβγ-mediated activation of phosphoinositide 3-kinase γ. Authors: Chun-Liang Chen / Ramizah Syahirah / Sandeep K Ravala / Yu-Chen Yen / Thomas Klose / Qing Deng / John J G Tesmer /  Abstract: The conversion of phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5-triphosphate by phosphoinositide 3-kinase γ (PI3Kγ) is critical for neutrophil chemotaxis and cancer metastasis. ...The conversion of phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5-triphosphate by phosphoinositide 3-kinase γ (PI3Kγ) is critical for neutrophil chemotaxis and cancer metastasis. PI3Kγ is activated by Gβγ heterodimers released from G protein-coupled receptors responding to extracellular signals. Here we determined cryo-electron microscopy structures of Sus scrofa PI3Kγ-human Gβγ complexes in the presence of substrates/analogs, revealing two Gβγ binding sites: one on the p110γ helical domain and another on the p101 C-terminal domain. Comparison with PI3Kγ alone reveals conformational changes in the kinase domain upon Gβγ binding that are similar to Ras·GTP-induced changes. Assays of variants perturbing the Gβγ binding sites and interdomain contacts altered by Gβγ binding suggest that Gβγ recruits the enzyme to membranes and allosterically regulates activity via both sites. Studies of zebrafish neutrophil migration align with these findings, paving the way for in-depth investigation of Gβγ-mediated activation mechanisms in this enzyme family and drug development for PI3Kγ. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40655.map.gz emd_40655.map.gz | 113.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40655-v30.xml emd-40655-v30.xml emd-40655.xml emd-40655.xml | 27 KB 27 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40655_fsc.xml emd_40655_fsc.xml | 11.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_40655.png emd_40655.png | 122.5 KB | ||
| Filedesc metadata |  emd-40655.cif.gz emd-40655.cif.gz | 8.4 KB | ||
| Others |  emd_40655_half_map_1.map.gz emd_40655_half_map_1.map.gz emd_40655_half_map_2.map.gz emd_40655_half_map_2.map.gz | 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40655 http://ftp.pdbj.org/pub/emdb/structures/EMD-40655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40655 | HTTPS FTP |
-Related structure data
| Related structure data |  8soeMC  8so9C  8soaC  8sobC  8socC  8sodC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40655.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40655.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_40655_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
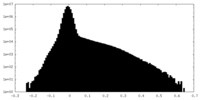
| Projections & Slices |
| ||||||||||||
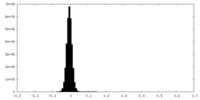
| Density Histograms |
-Half map: #1
| File | emd_40655_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
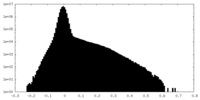
| Projections & Slices |
| ||||||||||||
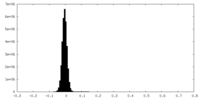
| Density Histograms |
- Sample components
Sample components
-Entire : PI3K-gamma-ADP bound with two Gbetagamma subunits in State 2
| Entire | Name: PI3K-gamma-ADP bound with two Gbetagamma subunits in State 2 |
|---|---|
| Components |
|
-Supramolecule #1: PI3K-gamma-ADP bound with two Gbetagamma subunits in State 2
| Supramolecule | Name: PI3K-gamma-ADP bound with two Gbetagamma subunits in State 2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Sus scrofa (pig) Sus scrofa (pig) |
| Molecular weight | Theoretical: 210 kDa/nm |
-Macromolecule #1: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Bos taurus (cattle) Bos taurus (cattle) |
| Molecular weight | Theoretical: 37.41693 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #2: phosphatidylinositol-4,5-bisphosphate 3-kinase
| Macromolecule | Name: phosphatidylinositol-4,5-bisphosphate 3-kinase / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Sus scrofa (pig) Sus scrofa (pig) |
| Molecular weight | Theoretical: 127.573531 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MHHHHHHELE NYEQPVVLRE DNRRRRRRMK PRSTAASLSS MELIPIEFVL PTSQRNTKTP ETALLHVAGH GNVEQMKAQV WLRALETSV SADFYHRLGP DHFLLLYQKK GQWYEIYDKY QVVQTLDCLR YWKVLHRSPG QIHVVQRHAP SEETLAFQRQ L NALIGYDV ...String: MHHHHHHELE NYEQPVVLRE DNRRRRRRMK PRSTAASLSS MELIPIEFVL PTSQRNTKTP ETALLHVAGH GNVEQMKAQV WLRALETSV SADFYHRLGP DHFLLLYQKK GQWYEIYDKY QVVQTLDCLR YWKVLHRSPG QIHVVQRHAP SEETLAFQRQ L NALIGYDV TDVSNVHDDE LEFTRRRLVT PRMAEVAGRD PKLYAMHPWV TSKPLPEYLL KKITNNCVFI VIHRSTTSQT IK VSADDTP GTILQSFFTK MAKKKSLMDI PESQNERDFV LRVCGRDEYL VGETPIKNFQ WVRQCLKNGE EIHLVLDTPP DPA LDEVRK EEWPLVDDCT GVTGYHEQLT IHGKDHESVF TVSLWDCDRK FRVKIRGIDI PVLPRTADLT VFVEANIQYG QQVL CQRRT SPKPFTEEVL WNVWLEFSIK IKDLPKGALL NLQIYCGKAP ALSGKTSAEM PSPESKGKAQ LLYYVNLLLI DHRFL LRHG EYVLHMWQLS GKGEDQGSFN ADKLTSATNP DKENSMSISI LLDNYCHPIA LPKHRPTPDP EGDRVRAEMP NQLRKQ LEA IIATDPLNPL TAEDKELLWH FRYESLKDPK AYPKLFSSVK WGQQEIVAKT YQLLAKREVW DQSALDVGLT MQLLDCN FS DENVRAIAVQ KLESLEDDDV LHYLLQLVQA VKFEPYHDSA LARFLLKRGL RNKRIGHFLF WFLRSEIAQS RHYQQRFA V ILEAYLRGCG TAMLHDFTQQ VQVIDMLQKV TIDIKSLSAE KYDVSSQVIS QLKQKLENLQ NLNLPQSFRV PYDPGLKAG ALVIEKCKVM ASKKKPLWLE FKCADPTALS NETIGIIFKH GDDLRQDMLI LQILRIMESI WETESLDLCL LPYGCISTGD KIGMIEIVK DATTIAKIQQ STVGNTGAFK DEVLSHWLKE KCPIEEKFQA AVERFVYSCA GYCVATFVLG IGDRHNDNIM I SETGNLFH IDFGHILGNY KSFLGINKER VPFVLTPDFL FVMGTSGKKT SLHFQKFQDV CVKAYLALRH HTNLLIILFS MM LMTGMPQ LTSKEDIEYI RDALTVGKSE EDAKKYFLDQ IEVCRDKGWT VQFNWFLHLV LGIKQGEKHS A UniProtKB:  phosphatidylinositol-4,5-bisphosphate 3-kinase phosphatidylinositol-4,5-bisphosphate 3-kinase |
-Macromolecule #3: Phosphoinositide 3-kinase regulatory subunit 5
| Macromolecule | Name: Phosphoinositide 3-kinase regulatory subunit 5 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Sus scrofa (pig) Sus scrofa (pig) |
| Molecular weight | Theoretical: 98.497773 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MQPGATTCTE DRIQHALERC LHGLSLSRRS TSWSAGLCLN CWSLQELVSR DPGHFLILLE QILQKTREVQ EKGTYDLLAP LALLFYSTV LCTPHFPPDS DLLLKAARTY HRFLTWPVPY CSICQELLTF IDAELKAPGI SYQRLVRAEQ GLSTRSHRSS T VTVLLLNP ...String: MQPGATTCTE DRIQHALERC LHGLSLSRRS TSWSAGLCLN CWSLQELVSR DPGHFLILLE QILQKTREVQ EKGTYDLLAP LALLFYSTV LCTPHFPPDS DLLLKAARTY HRFLTWPVPY CSICQELLTF IDAELKAPGI SYQRLVRAEQ GLSTRSHRSS T VTVLLLNP VEVQAEFLDV ADKLSTPGPS PHSAYITLLL HAFQATFGAH CDLSGLHRRL QSKTLAELEA IFTETAEAQE LA SGIGDAA EARQWLRTKL QAVGEKAGFP GVLDTAKPGK LRTIPIPVAR CYTYSWNQDS FDILQEILLK EQELLQPEIL DDE EDEDEE DEEEDLDADG HCAERDSVLS TGSAASHAST LSLASSQASG PTLSRQLLTS FVSGLSDGVD SGYMEDIEES AYER PRRPG GHERRGHRRP GQKFNRIYKL FKSTSQMVLR RDSRSLEGSP DSGPPLRRAG SLCSPLDSPT LPPSRAQRSR SLPQP KLSP QLPGWLLAPA SRHQRRRPFL SGDEDPKAST LRVVVFGSDR ISGKVARAYS NLRRLENNRP LLTRFFKLQF FYVPVK RSR GTGTPTSPAP RSQTPPLPTD APRHPGPAEL GAAPWEESTN DISHYLGMLD PWYERNVLGL MHLPPEVLCQ SLKAEPR PL EGSPAQLPIL ADMLLYYCRF AARPVLLQVY QTELTFITGE KTTEIFIHSL ELGHSAATRA IKASGPGSKR LGIDGDRE A VPLTLQIIYS KGAISGRSRW SNMEKLCTSV NLSKACRQQE ELDSSTEALT LNLTEVVKRQ TPKSKKGFNQ ISTSQIKVD KVQIIGSNSC PFAVCLDQDE RKILQSVIRC EVSPCYKPEK SSLCPPPQRP SYPPAPATPD LCSLLCLPIM TFSGALPGGG GSDYKDDDD K UniProtKB: Phosphoinositide 3-kinase regulatory subunit 5 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Bos taurus (cattle) Bos taurus (cattle) |
| Molecular weight | Theoretical: 8.673959 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: HHHHHHMASN NTASIAQARK LVEQLKMEAN IDRIKVSKAA ADLMAYCEAH AKEDPLLTPV PASENPFREK KFFSAIL UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Support film - Material: GOLD / Support film - topology: HOLEY | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Blot force 2. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Specialist optics | Energy filter - Name: GIF Quantum ER / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Alignment procedure | Coma free - Residual tilt: 0.01 mrad |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 11520 pixel / Digitization - Dimensions - Height: 8184 pixel / Average exposure time: 3.12 sec. / Average electron dose: 55.0 e/Å2 Details: Images were collected in movie-mode at 40 frames per second |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Target criteria: correlation coefficient | ||||||||||
| Output model |  PDB-8soe: |
 Movie
Movie Controller
Controller



















 Z
Z Y
Y X
X