+ Open data
Open data
- Basic information
Basic information
| Entry | 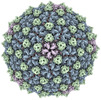 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mycobacterium phage Adjutor | |||||||||
 Map data Map data | Sharpened map of ewald sphere corrected postprocess. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | T=7 /  HK97 / HK97 /  Tailed bacteriophage / Tailed bacteriophage /  Capsid / Capsid /  VIRUS VIRUS | |||||||||
| Function / homology | Uncharacterized protein / Capsid decoration protein / Major capsid protein Function and homology information Function and homology information | |||||||||
| Biological species |  Mycobacterium phage Adjutor (virus) Mycobacterium phage Adjutor (virus) | |||||||||
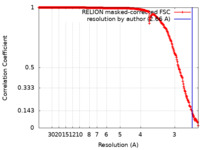
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.66 Å cryo EM / Resolution: 2.66 Å | |||||||||
 Authors Authors | Podgorski JM / White SJ | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: A novel accessory protein stabilizes the capsid of two actinobacteriophages Authors: Podgorski JM / Podgorski J / Gosselin S / Abad L / Jacobs-Sera D / Brown C / Hatfull G / Gogarten P / Luque A / White SJ | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40271.map.gz emd_40271.map.gz | 1.7 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40271-v30.xml emd-40271-v30.xml emd-40271.xml emd-40271.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40271_fsc.xml emd_40271_fsc.xml | 27.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_40271.png emd_40271.png | 293 KB | ||
| Masks |  emd_40271_msk_1.map emd_40271_msk_1.map | 1.9 GB |  Mask map Mask map | |
| Filedesc metadata |  emd-40271.cif.gz emd-40271.cif.gz | 6 KB | ||
| Others |  emd_40271_additional_1.map.gz emd_40271_additional_1.map.gz emd_40271_half_map_1.map.gz emd_40271_half_map_1.map.gz emd_40271_half_map_2.map.gz emd_40271_half_map_2.map.gz | 1.8 GB 1.5 GB 1.5 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40271 http://ftp.pdbj.org/pub/emdb/structures/EMD-40271 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40271 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40271 | HTTPS FTP |
-Related structure data
| Related structure data |  8sajMC  8giuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40271.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40271.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map of ewald sphere corrected postprocess. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.2 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
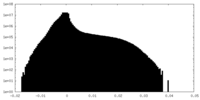
| File |  emd_40271_msk_1.map emd_40271_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Ewald sphere corrected map.
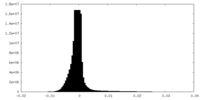
| File | emd_40271_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Ewald sphere corrected map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of ewald sphere corrected map.
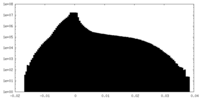
| File | emd_40271_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of ewald sphere corrected map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of ewald sphere corrected map.
| File | emd_40271_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of ewald sphere corrected map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Mycobacterium phage Adjutor
| Entire | Name:  Mycobacterium phage Adjutor (virus) Mycobacterium phage Adjutor (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Mycobacterium phage Adjutor
| Supramolecule | Name: Mycobacterium phage Adjutor / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 528321 / Sci species name: Mycobacterium phage Adjutor / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Virus shell | Shell ID: 1 / Diameter: 750.0 Å / T number (triangulation number): 7 |
-Macromolecule #1: Major capsid protein
| Macromolecule | Name: Major capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium phage Adjutor (virus) Mycobacterium phage Adjutor (virus) |
| Molecular weight | Theoretical: 45.525645 KDa |
| Sequence | String: MKTATEGRVM RESFLEIIAV VGLSGHDNQG GYNTAGDIKY KTADGVSYDS LWNLFSNVTD EWNKHKSKMV QLMTFPVTNQ TEKVPRIGQ FGFEKASEFG VPESKRTELS FYQLAYDFED YDLAFRYTWK FLRDAPSSQI KAYHNQALQA DAKLIHRKVM E AIFDNRER ...String: MKTATEGRVM RESFLEIIAV VGLSGHDNQG GYNTAGDIKY KTADGVSYDS LWNLFSNVTD EWNKHKSKMV QLMTFPVTNQ TEKVPRIGQ FGFEKASEFG VPESKRTELS FYQLAYDFED YDLAFRYTWK FLRDAPSSQI KAYHNQALQA DAKLIHRKVM E AIFDNRER EADIEGLPYK VYPLYNGDNM IPPEYNGTTF STGHNHYLVS GGTKIDSADV EMAADHIREH GYTEENGTQL IA FAHKAEI QEVRRFRFGQ TNNNSAVANY DFVQSQGESP LYLPNADGLL GKQPQSMWKG LRVKGSYDDV LWIEEPTMPA GYV LFLATG GTLAQQNLVG LREHEDAAWR GLRQIPGNQT RYPLIDSFYQ RSFGTGIRQR GGAVVLQIKA SGTYDIPTKW TNGG GFE UniProtKB: Major capsid protein |
-Macromolecule #2: gp_16 (Minor Capsid Protein)
| Macromolecule | Name: gp_16 (Minor Capsid Protein) / type: protein_or_peptide / ID: 2 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium phage Adjutor (virus) Mycobacterium phage Adjutor (virus) |
| Molecular weight | Theoretical: 13.766352 KDa |
| Sequence | String: MARYDKYNPY GGGFRAPLAA DWTDADAGKL YAVGINNVGA VVKGAGQSGV AGVLVLTKGA KAGSIVDVMK FGEVVEFGPT SGTPGTDFG AAGTAYYADT STGAINSTSG EAKVKVGHTV GAQRLIVAVA DGVVDPSPAA UniProtKB: Capsid decoration protein |
-Macromolecule #3: HNH endonuclease
| Macromolecule | Name: HNH endonuclease / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium phage Adjutor (virus) Mycobacterium phage Adjutor (virus) |
| Molecular weight | Theoretical: 6.198103 KDa |
| Sequence | String: MAKGVKKLPK RKGTNPIPRD KWNSDDIARR QLEQDQKLHL TTKGPHTGTN DSFK UniProtKB: Uncharacterized protein |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: COUNTING / Number real images: 6664 / Average electron dose: 30.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Details | Amino acid sequence built into the map for a single major capsid protein and refined with Phenix. Model then used for rest of asymmetric unit and refined with Phenix. Final step involved using Isolde. |
|---|---|
| Refinement | Protocol: AB INITIO MODEL |
| Output model |  PDB-8saj: |
 Movie
Movie Controller
Controller




 Z
Z Y
Y X
X