[English] 日本語
 Yorodumi
Yorodumi- EMDB-38011: Cryo-EM structure of the TcsL at pH 5.0 in its open conformation -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the TcsL at pH 5.0 in its open conformation | |||||||||
 Map data Map data | Author stated: the relative low model inclusion of map was due to the flexible mobile nature of the molecule. We modeled the C-terminus of the TcsL by rigid docking in to the low-pass filtered map. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TcsL / CROP /  TOXIN TOXIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell cytosol /  Transferases; Glycosyltransferases; Hexosyltransferases / Transferases; Glycosyltransferases; Hexosyltransferases /  glycosyltransferase activity / cysteine-type peptidase activity / host cell endosome membrane / glycosyltransferase activity / cysteine-type peptidase activity / host cell endosome membrane /  toxin activity / toxin activity /  Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases /  lipid binding / host cell plasma membrane / lipid binding / host cell plasma membrane /  proteolysis ...host cell cytosol / proteolysis ...host cell cytosol /  Transferases; Glycosyltransferases; Hexosyltransferases / Transferases; Glycosyltransferases; Hexosyltransferases /  glycosyltransferase activity / cysteine-type peptidase activity / host cell endosome membrane / glycosyltransferase activity / cysteine-type peptidase activity / host cell endosome membrane /  toxin activity / toxin activity /  Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases /  lipid binding / host cell plasma membrane / lipid binding / host cell plasma membrane /  proteolysis / extracellular region / proteolysis / extracellular region /  membrane / membrane /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||
| Biological species |  Paeniclostridium sordellii (strain ATCC 9714 / DSM 2141 / JCM 3814 / LMG 15708 / NCIMB 10717 / 211) (bacteria) Paeniclostridium sordellii (strain ATCC 9714 / DSM 2141 / JCM 3814 / LMG 15708 / NCIMB 10717 / 211) (bacteria) | |||||||||
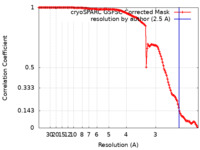
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.5 Å cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Zhan X / Tao L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural dynamics of CROPs control stability and toxicity of Paeniclostridium sordellii lethal toxin Authors: Yao Z / Xiechao Z / Liang T | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38011.map.gz emd_38011.map.gz | 230.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38011-v30.xml emd-38011-v30.xml emd-38011.xml emd-38011.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_38011_fsc.xml emd_38011_fsc.xml | 13.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_38011.png emd_38011.png | 38.3 KB | ||
| Filedesc metadata |  emd-38011.cif.gz emd-38011.cif.gz | 6.6 KB | ||
| Others |  emd_38011_half_map_1.map.gz emd_38011_half_map_1.map.gz emd_38011_half_map_2.map.gz emd_38011_half_map_2.map.gz | 226.4 MB 226.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38011 http://ftp.pdbj.org/pub/emdb/structures/EMD-38011 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38011 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38011 | HTTPS FTP |
-Related structure data
| Related structure data |  8x2iMC  8jb5C  8x2hC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
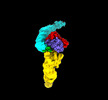
Map
| File |  Download / File: emd_38011.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38011.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Author stated: the relative low model inclusion of map was due to the flexible mobile nature of the molecule. We modeled the C-terminus of the TcsL by rigid docking in to the low-pass filtered map. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.087 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_38011_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
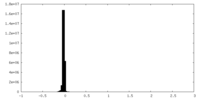
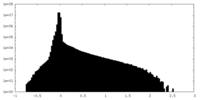
| Density Histograms |
-Half map: #2
| File | emd_38011_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
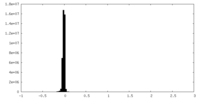
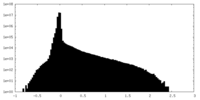
| Density Histograms |
- Sample components
Sample components
-Entire : TcsL
| Entire | Name: TcsL |
|---|---|
| Components |
|
-Supramolecule #1: TcsL
| Supramolecule | Name: TcsL / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Paeniclostridium sordellii (strain ATCC 9714 / DSM 2141 / JCM 3814 / LMG 15708 / NCIMB 10717 / 211) (bacteria) Paeniclostridium sordellii (strain ATCC 9714 / DSM 2141 / JCM 3814 / LMG 15708 / NCIMB 10717 / 211) (bacteria) |
-Macromolecule #1: Cytotoxin-L
| Macromolecule | Name: Cytotoxin-L / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number:  Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases |
|---|---|
| Source (natural) | Organism:  Paeniclostridium sordellii (strain ATCC 9714 / DSM 2141 / JCM 3814 / LMG 15708 / NCIMB 10717 / 211) (bacteria) Paeniclostridium sordellii (strain ATCC 9714 / DSM 2141 / JCM 3814 / LMG 15708 / NCIMB 10717 / 211) (bacteria) |
| Molecular weight | Theoretical: 271.827531 KDa |
| Recombinant expression | Organism:   Bacillus subtilis (bacteria) Bacillus subtilis (bacteria) |
| Sequence | String: MNLVNKAQLQ KMAYVKFRIQ EDEYVAILNA LEEYHNMSES SVVEKYLKLK DINNLTDNYL NTYKKSGRNK ALKKFKEYLT MEVLELKNN SLTPVEKNLH FIWIGGQIND TAINYINQWK DVNSDYTVKV FYDSNAFLIN TLKKTIVESA TNNTLESFRE N LNDPEFDY ...String: MNLVNKAQLQ KMAYVKFRIQ EDEYVAILNA LEEYHNMSES SVVEKYLKLK DINNLTDNYL NTYKKSGRNK ALKKFKEYLT MEVLELKNN SLTPVEKNLH FIWIGGQIND TAINYINQWK DVNSDYTVKV FYDSNAFLIN TLKKTIVESA TNNTLESFRE N LNDPEFDY NKFYRKRMEI IYDKQKHFID YYKSQIEENP EFIIDNIIKT YLSNEYSKDL EALNKYIEES LNKITANNGN DI RNLEKFA DEDLVRLYNQ ELVERWNLAA ASDILRISML KEDGGVYLDV DMLPGIQPDL FKSINKPDSI TNTSWEMIKL EAI MKYKEY IPGYTSKNFD MLDEEVQRSF ESALSSKSDK SEIFLPLDDI KVSPLEVKIA FANNSVINQA LISLKDSYCS DLVI NQIKN RYKILNDNLN PSINEGTDFN TTMKIFSDKL ASISNEDNMM FMIKITNYLK VGFAPDVRST INLSGPGVYT GAYQD LLMF KDNSTNIHLL EPELRNFEFP KTKISQLTEQ EITSLWSFNQ ARAKSQFEEY KKGYFEGALG EDDNLDFAQN TVLDKD YVS KKILSSMKTR NKEYIHYIVQ LQGDKISYEA SCNLFSKDPY SSILYQKNIE GSETAYYYSV ADAEIKEIDK YRIPYQI SN KRKIKLTFIG HGKSEFNTDT FANLDVDSLS SEIETILNLA KADISPKYIE INLLGCNMFS YSISAEETYP GKLLLKIK D RVSELMPSIS QDSITVSANQ YEVRINEEGK REILDHSGKW INKEESIIKD ISSKEYISFN PKENKIIVKS KYLHELSTL LQEIRNNANS SDIDLEKKVM LTECEINVAS NIDRQIVEGR IEEAKNLTSD SINYIKNEFK LIESISDSLY DLKHQNGLDD SHFISFEDI SKTENGFRIR FINKETGNSI FIETEKEIFS EYATHISKEI SNIKDTIFDN VNGKLVKKVN LDAAHEVNTL N SAFFIQSL IEYNTTKESL SNLSVAMKVQ VYAQLFSTGL NTITDASKVV ELVSTALDET IDLLPTLSEG LPIIATIIDG VS LGAAIKE LSETNDPLLR QEIEAKIGIM AVNLTAASTA IVTSALGIAS GFSILLVPLA GISAGIPSLV NNELILQDKA TKV IDYFKH ISLAETEGAF TLLDDKIIMP QDDLVLSEID FNNNSITLGK CEIWRAEGGS GHTLTDDIDH FFSSPSITYR KPWL SIYDV LNIKKEKIDF SKDLMVLPNA PNRVFGYEMG WTPGFRSLDN DGTKLLDRIR DHYEGQFYWR YFAFIADALI TKLKP RYED TNVRINLDGN TRSFIVPVIT TEQIRKNLSY SFYGSGGSYS LSLSPYNMNI DLNLVENDTW VIDVDNVVKN ITIESD EIQ KGELIENILS KLNIEDNKII LNNHTINFYG DINESNRFIS LTFSILEDIN IIIEIDLVSK SYKILLSGNC MKLIENS SD IQQKIDHIGF NGEHQKYIPY SYIDNETKYN GFIDYSKKEG LFTAEFSNES IIRNIYMPDS NNLFIYSSKD LKDIRIIN K GDVKLLIGNY FKDDMKVSLS FTIEDTNTIK LNGVYLDENG VAQILKFMNN AKSALNTSNS LMNFLESINI KNIFYNNLD PNIEFILDTN FIISGSNSIG QFELICDKDK NIQPYFIKFK IKETSYTLYV GNRQNLIVEP SYHLDDSGNI SSTVINFSQK YLYGIDRYV NKVIIAPNLY TDEINITPVY KPNYICPEVI ILDANYINEK INVNINDLSI RYVWDNDGSD LILIANSEED N QPQVKIRF VNVFKSDTAA DKLSFNFSDK QDVSVSKIIS TFSLAAYSDG FFDYEFGLVS LDNDYFYINS FGNMVSGLIY IN DSLYYFK PPKNNLITGF TTIDGNKYYF DPTKSGAASI GEITIDGKDY YFNKQGILQV GVINTSDGLK YFAPAGTLDE NLE GESVNF IGKLNIDGKI YYFEDNYRAA VEWKLLDDET YYFNPKTGEA LKGLHQIGDN KYYFDDNGIM QTGFITINDK VFYF NNDGV MQVGYIEVNG KYFYFGKNGE RQLGVFNTPD GFKFFGPKDD DLGTEEGELT LYNGILNFNG KIYFFDISNT AVVGW GTLD DGSTYYFDDN TAEACIGLTV INDCKYYFDD NGIRQLGFIT INDNIFYFSE SGKIELGYQN INGNYFYIDE SGLVLI GVF DTPDGYKYFA PLNTVNDNIY GQAVKYSGLV RVNEDVYYFG ETYKIETGWI ENETDKYYFD PETKKAYKGI NVVDDIK YY FDENGIMRTG LISFENNNYY FNEDGKMQFG YLNIKDKMFY FGKDGKMQIG VFNTPDGFKY FAHQNTLDEN FEGESINY T GWLDLDGKRY YFTDEYIAAT GSLTIDGYNY YFDPDTAELV VSEHHHHHHH H UniProtKB: Cytotoxin-L |
-Macromolecule #2: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 2 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.5 µm Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.5 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X