[English] 日本語
 Yorodumi
Yorodumi- EMDB-37675: Cryo EM map of SLC7A10-SLC3A2 complex in the D-serine bound state -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo EM map of SLC7A10-SLC3A2 complex in the D-serine bound state | |||||||||
 Map data Map data | cryo EM map of SLC7A10-SLC3A2 complex in the D-serine bound state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SLC7A10 / SLC3A2 /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationD-alanine transmembrane transport / D-serine transmembrane transport / positive regulation of synaptic transmission, glycinergic / glycine transport / L-serine transmembrane transporter activity / neutral L-amino acid secondary active transmembrane transporter activity / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / methionine transport / tyrosine transport / L-histidine transport ...D-alanine transmembrane transport / D-serine transmembrane transport / positive regulation of synaptic transmission, glycinergic / glycine transport / L-serine transmembrane transporter activity / neutral L-amino acid secondary active transmembrane transporter activity / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / methionine transport / tyrosine transport / L-histidine transport / apical pole of neuron / aromatic amino acid transmembrane transporter activity / amino acid transport complex / L-amino acid transmembrane transporter activity / : / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity / isoleucine transport / L-alanine import across plasma membrane / phenylalanine transport / valine transport / negative regulation of brown fat cell differentiation / L-leucine transmembrane transporter activity / proline transport / amino acid transmembrane transport / calcium:sodium antiporter activity / L-leucine transport / thyroid hormone transport / neutral amino acid transport / Amino acid transport across the plasma membrane / neutral L-amino acid transmembrane transporter activity / Tryptophan catabolism /  exogenous protein binding / amino acid transport / anchoring junction / Basigin interactions / response to exogenous dsRNA / tryptophan transport / basal plasma membrane / calcium ion transport / exogenous protein binding / amino acid transport / anchoring junction / Basigin interactions / response to exogenous dsRNA / tryptophan transport / basal plasma membrane / calcium ion transport /  double-stranded RNA binding / double-stranded RNA binding /  melanosome / virus receptor activity / basolateral plasma membrane / carbohydrate metabolic process / melanosome / virus receptor activity / basolateral plasma membrane / carbohydrate metabolic process /  cadherin binding / symbiont entry into host cell / apical plasma membrane / protein heterodimerization activity / lysosomal membrane / cadherin binding / symbiont entry into host cell / apical plasma membrane / protein heterodimerization activity / lysosomal membrane /  synapse / synapse /  cell surface / protein homodimerization activity / cell surface / protein homodimerization activity /  RNA binding / extracellular exosome / RNA binding / extracellular exosome /  nucleoplasm / nucleoplasm /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.5 Å cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Li YN / Guo YY / Dai L / Yan RH | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Cryo-EM structure of the human Asc-1 transporter complex. Authors: Yaning Li / Yingying Guo / Angelika Bröer / Lu Dai / Stefan Brӧer / Renhong Yan /   Abstract: The Alanine-Serine-Cysteine transporter 1 (Asc-1 or SLC7A10) forms a crucial heterodimeric transporter complex with 4F2hc (SLC3A2) through a covalent disulfide bridge. This complex enables the sodium- ...The Alanine-Serine-Cysteine transporter 1 (Asc-1 or SLC7A10) forms a crucial heterodimeric transporter complex with 4F2hc (SLC3A2) through a covalent disulfide bridge. This complex enables the sodium-independent transport of small neutral amino acids, including L-Alanine (L-Ala), Glycine (Gly), and D-Serine (D-Ser), within the central nervous system (CNS). D-Ser and Gly are two key endogenous glutamate co-agonists that activate N-methyl-d-aspartate (NMDA) receptors by binding to the allosteric site. Mice deficient in Asc-1 display severe symptoms such as tremors, ataxia, and seizures, leading to early postnatal death. Despite its physiological importance, the functional mechanism of the Asc-1-4F2hc complex has remained elusive. Here, we present cryo-electron microscopy (cryo-EM) structures of the human Asc-1-4F2hc complex in its apo state, D-Ser bound state, and L-Ala bound state, resolved at 3.6 Å, 3.5 Å, and 3.4 Å, respectively. Through detailed structural analysis and transport assays, we uncover a comprehensive alternating access mechanism that underlies conformational changes in the complex. In summary, our findings reveal the architecture of the Asc-1 and 4F2hc complex and provide valuable insights into substrate recognition and the functional cycle of this essential transporter complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37675.map.gz emd_37675.map.gz | 59.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37675-v30.xml emd-37675-v30.xml emd-37675.xml emd-37675.xml | 15.6 KB 15.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37675.png emd_37675.png | 55.1 KB | ||
| Filedesc metadata |  emd-37675.cif.gz emd-37675.cif.gz | 6 KB | ||
| Others |  emd_37675_half_map_1.map.gz emd_37675_half_map_1.map.gz emd_37675_half_map_2.map.gz emd_37675_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37675 http://ftp.pdbj.org/pub/emdb/structures/EMD-37675 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37675 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37675 | HTTPS FTP |
-Related structure data
| Related structure data |  8wnyMC  8wnsC  8wntC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37675.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37675.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo EM map of SLC7A10-SLC3A2 complex in the D-serine bound state | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.095 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: cryo EM half map of SLC7A10-SLC3A2 complex in...
| File | emd_37675_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo EM half map of SLC7A10-SLC3A2 complex in the D-serine bound state | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: cryo EM half map of SLC7A10-SLC3A2 complex in...
| File | emd_37675_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo EM half map of SLC7A10-SLC3A2 complex in the D-serine bound state | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : 4F2hc-ASC-1 complex
| Entire | Name: 4F2hc-ASC-1 complex |
|---|---|
| Components |
|
-Supramolecule #1: 4F2hc-ASC-1 complex
| Supramolecule | Name: 4F2hc-ASC-1 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Amino acid transporter heavy chain SLC3A2
| Macromolecule | Name: Amino acid transporter heavy chain SLC3A2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 68.16468 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MELQPPEASI AVVSIPRQLP GSHSEAGVQG LSAGDDSETG SDCVTQAGLQ LLASSDPPAL ASKNAEVTVE TGFHHVSQAD IEFLTSIDP TASASGSAGI TGTMSQDTEV DMKEVELNEL EPEKQPMNAA SGAAMSLAGA EKNGLVKIKV AEDEAEAAAA A KFTGLSKE ...String: MELQPPEASI AVVSIPRQLP GSHSEAGVQG LSAGDDSETG SDCVTQAGLQ LLASSDPPAL ASKNAEVTVE TGFHHVSQAD IEFLTSIDP TASASGSAGI TGTMSQDTEV DMKEVELNEL EPEKQPMNAA SGAAMSLAGA EKNGLVKIKV AEDEAEAAAA A KFTGLSKE ELLKVAGSPG WVRTRWALLL LFWLGWLGML AGAVVIIVRA PRCRELPAQK WWHTGALYRI GDLQAFQGHG AG NLAGLKG RLDYLSSLKV KGLVLGPIHK NQKDDVAQTD LLQIDPNFGS KEDFDSLLQS AKKKSIRVIL DLTPNYRGEN SWF STQVDT VATKVKDALE FWLQAGVDGF QVRDIENLKD ASSFLAEWQN ITKGFSEDRL LIAGTNSSDL QQILSLLESN KDLL LTSSY LSDSGSTGEH TKSLVTQYLN ATGNRWCSWS LSQARLLTSF LPAQLLRLYQ LMLFTLPGTP VFSYGDEIGL DAAAL PGQP MEAPVMLWDE SSFPDIPGAV SANMTVKGQS EDPGSLLSLF RRLSDQRSKE RSLLHGDFHA FSAGPGLFSY IRHWDQ NER FLVVLNFGDV GLSAGLQASD LPASASLPAK ADLLLSTQPG REEGSPLELE RLKLEPHEGL LLRFPYAA UniProtKB: Amino acid transporter heavy chain SLC3A2 |
-Macromolecule #2: Asc-type amino acid transporter 1
| Macromolecule | Name: Asc-type amino acid transporter 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.837504 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAGHTQQPSG RGNPRPAPSP SPVPGTVPGA SERVALKKEI GLLSACTIII GNIIGSGIFI SPKGVLEHSG SVGLALFVWV LGGGVTALG SLCYAELGVA IPKSGGDYAY VTEIFGGLAG FLLLWSAVLI MYPTSLAVIS MTFSNYVLQP VFPNCIPPTT A SRVLSMAC ...String: MAGHTQQPSG RGNPRPAPSP SPVPGTVPGA SERVALKKEI GLLSACTIII GNIIGSGIFI SPKGVLEHSG SVGLALFVWV LGGGVTALG SLCYAELGVA IPKSGGDYAY VTEIFGGLAG FLLLWSAVLI MYPTSLAVIS MTFSNYVLQP VFPNCIPPTT A SRVLSMAC LMLLTWVNSS SVRWATRIQD MFTGGKLLAL SLIIGVGLLQ IFQGHFEELR PSNAFAFWMT PSVGHLALAF LQ GSFAFSG WNFLNYVTEE MVDARKNLPR AIFISIPLVT FVYTFTNIAY FTAMSPQELL SSNAVAVTFG EKLLGYFSWV MPV SVALST FGGINGYLFT YSRLCFSGAR EGHLPSLLAM IHVRHCTPIP ALLVCCGATA VIMLVGDTYT LINYVSFINY LCYG VTILG LLLLRWRRPA LHRPIKVNLL IPVAYLVFWA FLLVFSFISE PMVCGVGVII ILTGVPIFFL GVFWRSKPKC VHRLT ESMT HWGQELCFVV YPQDAPEEEE NGPCPPSLLP ATDKPSKPQ UniProtKB: Asc-type amino acid transporter 1 |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 4 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: D-SERINE
| Macromolecule | Name: D-SERINE / type: ligand / ID: 4 / Number of copies: 1 / Formula: DSN |
|---|---|
| Molecular weight | Theoretical: 105.093 Da |
| Chemical component information | 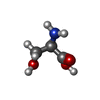 ChemComp-DSN: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 300000 |
 Movie
Movie Controller
Controller









 Z
Z Y
Y X
X

















