+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the GPR61-Gs complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  GPCR / Gs / GPCR / Gs /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationligand-independent adenylate cyclase-activating G protein-coupled receptor signaling pathway / Adenylate cyclase activating pathway / arrestin family protein binding / PKA activation in glucagon signalling / hair follicle placode formation / Adenylate cyclase inhibitory pathway / developmental growth /  D1 dopamine receptor binding / D1 dopamine receptor binding /  intracellular transport / Hedgehog 'off' state ...ligand-independent adenylate cyclase-activating G protein-coupled receptor signaling pathway / Adenylate cyclase activating pathway / arrestin family protein binding / PKA activation in glucagon signalling / hair follicle placode formation / Adenylate cyclase inhibitory pathway / developmental growth / intracellular transport / Hedgehog 'off' state ...ligand-independent adenylate cyclase-activating G protein-coupled receptor signaling pathway / Adenylate cyclase activating pathway / arrestin family protein binding / PKA activation in glucagon signalling / hair follicle placode formation / Adenylate cyclase inhibitory pathway / developmental growth /  D1 dopamine receptor binding / D1 dopamine receptor binding /  intracellular transport / Hedgehog 'off' state / positive regulation of cAMP-mediated signaling / adenylate cyclase-activating adrenergic receptor signaling pathway / activation of adenylate cyclase activity / adenylate cyclase activator activity / trans-Golgi network membrane / G protein-coupled receptor binding / G protein-coupled receptor activity / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / intracellular transport / Hedgehog 'off' state / positive regulation of cAMP-mediated signaling / adenylate cyclase-activating adrenergic receptor signaling pathway / activation of adenylate cyclase activity / adenylate cyclase activator activity / trans-Golgi network membrane / G protein-coupled receptor binding / G protein-coupled receptor activity / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway /  bone development / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / adenylate cyclase-activating G protein-coupled receptor signaling pathway / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / bone development / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / adenylate cyclase-activating G protein-coupled receptor signaling pathway / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors /  platelet aggregation / platelet aggregation /  cognition / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of GTPase activity / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding / cognition / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of GTPase activity / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding /  heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events /  extracellular vesicle / signaling receptor complex adaptor activity / sensory perception of smell / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / extracellular vesicle / signaling receptor complex adaptor activity / sensory perception of smell / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye /  GTPase binding / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cold-induced thermogenesis / G alpha (i) signalling events / fibroblast proliferation / G alpha (s) signalling events / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / GTPase binding / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cold-induced thermogenesis / G alpha (i) signalling events / fibroblast proliferation / G alpha (s) signalling events / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling /  receptor complex / endosome membrane / receptor complex / endosome membrane /  endosome / G protein-coupled receptor signaling pathway / lysosomal membrane / endosome / G protein-coupled receptor signaling pathway / lysosomal membrane /  GTPase activity / GTPase activity /  synapse / protein-containing complex binding / GTP binding / synapse / protein-containing complex binding / GTP binding /  signal transduction / extracellular exosome / signal transduction / extracellular exosome /  membrane / membrane /  metal ion binding / metal ion binding /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Lama glama (llama) Lama glama (llama) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.16 Å cryo EM / Resolution: 3.16 Å | |||||||||
 Authors Authors | Nie Y / Qiu Z / Zheng S | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Specific binding of GPR174 by endogenous lysophosphatidylserine leads to high constitutive G signaling. Authors: Yingying Nie / Zeming Qiu / Sijia Chen / Zhao Chen / Xiaocui Song / Yan Ma / Niu Huang / Jason G Cyster / Sanduo Zheng /   Abstract: Many orphan G protein-coupled receptors (GPCRs) remain understudied because their endogenous ligands are unknown. Here, we show that a group of class A/rhodopsin-like orphan GPCRs including GPR61, ...Many orphan G protein-coupled receptors (GPCRs) remain understudied because their endogenous ligands are unknown. Here, we show that a group of class A/rhodopsin-like orphan GPCRs including GPR61, GPR161 and GPR174 increase the cAMP level similarly to fully activated D1 dopamine receptor (D1R). We report cryo-electron microscopy structures of the GPR61‒G, GPR161‒G and GPR174‒G complexes without any exogenous ligands. The GPR174 structure reveals that endogenous lysophosphatidylserine (lysoPS) is copurified. While GPR174 fails to respond to exogenous lysoPS, likely owing to its maximal activation by the endogenous ligand, GPR174 mutants with lower ligand binding affinities can be specifically activated by lysoPS but not other lipids, in a dose-dependent manner. Moreover, GPR174 adopts a non-canonical G coupling mode. The structures of GPR161 and GPR61 reveal that the second extracellular loop (ECL2) penetrates into the orthosteric pocket, possibly contributing to constitutive activity. Our work definitively confirms lysoPS as an endogenous GPR174 ligand and suggests that high constitutive activity of some orphan GPCRs could be accounted for by their having naturally abundant ligands. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37224.map.gz emd_37224.map.gz | 20.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37224-v30.xml emd-37224-v30.xml emd-37224.xml emd-37224.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37224.png emd_37224.png | 68.5 KB | ||
| Filedesc metadata |  emd-37224.cif.gz emd-37224.cif.gz | 6.7 KB | ||
| Others |  emd_37224_half_map_1.map.gz emd_37224_half_map_1.map.gz emd_37224_half_map_2.map.gz emd_37224_half_map_2.map.gz | 20.7 MB 20.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37224 http://ftp.pdbj.org/pub/emdb/structures/EMD-37224 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37224 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37224 | HTTPS FTP |
-Related structure data
| Related structure data |  8kgkMC  8kh4C  8kh5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37224.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37224.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_37224_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
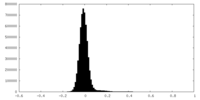
| Density Histograms |
-Half map: #2
| File | emd_37224_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
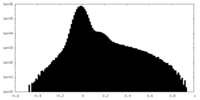
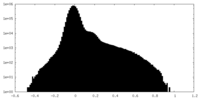
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of the GPR61 and mini-Gs complex with Nb35
| Entire | Name: Cryo-EM structure of the GPR61 and mini-Gs complex with Nb35 |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the GPR61 and mini-Gs complex with Nb35
| Supramolecule | Name: Cryo-EM structure of the GPR61 and mini-Gs complex with Nb35 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: GPR61 and mini-Gs
| Supramolecule | Name: GPR61 and mini-Gs / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Nb35
| Supramolecule | Name: Nb35 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #5 |
|---|---|
| Source (natural) | Organism:   Lama glama (llama) Lama glama (llama) |
-Macromolecule #1: G-protein coupled receptor 61
| Macromolecule | Name: G-protein coupled receptor 61 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.527605 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DYKDDDDKAM ESSPIPQSSG NSSTLGRVPQ TPGPSTASGV PEVGLRDVAS ESVALFFMLL LDLTAVAGNA AVMAVIAKTP ALRKFVFVF HLCLVDLLAA LTLMPLAMLS SSALFDHALF GEVACRLYLF LSVCFVSLAI LSVSAINVER YYYVVHPMRY E VRMTLGLV ...String: DYKDDDDKAM ESSPIPQSSG NSSTLGRVPQ TPGPSTASGV PEVGLRDVAS ESVALFFMLL LDLTAVAGNA AVMAVIAKTP ALRKFVFVF HLCLVDLLAA LTLMPLAMLS SSALFDHALF GEVACRLYLF LSVCFVSLAI LSVSAINVER YYYVVHPMRY E VRMTLGLV ASVLVGVWVK ALAMASVPVL GRVSWEEGAP SVPPGCSLQW SHSAYCQLFV VVFAVLYFLL PLLLILVVYC SM FRVARVA AMQHGPLPTW METPRQRSES LSSRSTMVTS SGAPQTTPHR TFGGGKAAVV LLAVGGQFLL CWLPYFSFHL YVA LSAQPI STGQVESVVT WIGYFCFTSN PFFYGCLNRQ IRGELSKQFV CFFKPAPELE VLFQGPLEVL FQGP UniProtKB:  G-protein coupled receptor 61 G-protein coupled receptor 61 |
-Macromolecule #2: Guanine nucleotide-binding protein G(olf) subunit alpha,Guanine n...
| Macromolecule | Name: Guanine nucleotide-binding protein G(olf) subunit alpha,Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 29.008785 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NSKTTEDQRN EEKAQREANK KIEKQLQKDK QVYRATHRLL LLGADNSGKS TIVKQMRILH GGSGGSGGTS GIFETKFQVD KVNFHMFDV GGQRDERRKW IQCFNDVTAI IFVVDSSDYN RLQEALNLFK SIWNNRWLRT ISVILFLNKQ DLLAEKVLAG K SKIEDYFP ...String: NSKTTEDQRN EEKAQREANK KIEKQLQKDK QVYRATHRLL LLGADNSGKS TIVKQMRILH GGSGGSGGTS GIFETKFQVD KVNFHMFDV GGQRDERRKW IQCFNDVTAI IFVVDSSDYN RLQEALNLFK SIWNNRWLRT ISVILFLNKQ DLLAEKVLAG K SKIEDYFP EFARYTTPED ATPEPGEDPR VTRAKYFIRD EFLRISTASG DGRHYCYPHF TCAVDTENAR RIFNDCRDII QR MHLRQYE LL UniProtKB: Guanine nucleotide-binding protein G(olf) subunit alpha, Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.286891 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: HHHHHHLEVL FQGPGSSGSE LDQLRQEAEQ LKNQIRDARK ACADATLSQI TNNIDPVGRI QMRTRRTLRG HLAKIYAMHW GTDSRLLVS ASQDGKLIIW DSYTTNKVHA IPLRSSWVMT CAYAPSGNYV ACGGLDNICS IYNLKTREGN VRVSRELAGH T GYLSCCRF ...String: HHHHHHLEVL FQGPGSSGSE LDQLRQEAEQ LKNQIRDARK ACADATLSQI TNNIDPVGRI QMRTRRTLRG HLAKIYAMHW GTDSRLLVS ASQDGKLIIW DSYTTNKVHA IPLRSSWVMT CAYAPSGNYV ACGGLDNICS IYNLKTREGN VRVSRELAGH T GYLSCCRF LDDNQIVTSS GDTTCALWDI ETGQQTTTFT GHTGDVMSLS LAPDTRLFVS GACDASAKLW DVREGMCRQT FT GHESDIN AICFFPNGNA FATGSDDATC RLFDLRADQE LMTYSHDNII CGITSVSFSK SGRLLLAGYD DFNCNVWDAL KAD RAGVLA GHDNRVSCLG VTDDGMAVAT GSWDSFLKIW N UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.845078 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFSAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: Nanobody 35
| Macromolecule | Name: Nanobody 35 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Lama glama (llama) Lama glama (llama) |
| Molecular weight | Theoretical: 17.352498 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: MKYLLPTAAA GLLLLAAQPA MAQVQLQESG GGLVQPGGSL RLSCAASGFT FSNYKMNWVR QAPGKGLEWV SDISQSGASI SYTGSVKGR FTISRDNAKN TLYLQMNSLK PEDTAVYYCA RCPAPFTRDC FDVTSTTYAY RGQGTQVTVS SHHHHHHEPE A |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7.0 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 35 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.48 µm / Nominal defocus min: 0.87 µm / Nominal magnification: 64000 Bright-field microscopy / Nominal defocus max: 2.48 µm / Nominal defocus min: 0.87 µm / Nominal magnification: 64000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 923 / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 277452 |
|---|---|
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final 3D classification | Number classes: 3 |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Number classes used: 1 / Resolution.type: BY AUTHOR / Resolution: 3.16 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 192672 |
 Movie
Movie Controller
Controller




























 Z
Z Y
Y X
X