+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3679 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
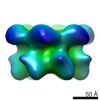
| Title | Full-length complex of S.cerevisiae Cdt1-MCM in apo state. | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 20.4 Å negative staining / Resolution: 20.4 Å | |||||||||
 Authors Authors | He J / Frigola J / Kinkelin K / Pye VE / Renault L / Douglas M / Remus D / Cherepanov P / Costa A / Diffley JFX | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2017 Journal: Nat Commun / Year: 2017Title: Cdt1 stabilizes an open MCM ring for helicase loading. Authors: Jordi Frigola / Jun He / Kerstin Kinkelin / Valerie E Pye / Ludovic Renault / Max E Douglas / Dirk Remus / Peter Cherepanov / Alessandro Costa / John F X Diffley /   Abstract: ORC, Cdc6 and Cdt1 act together to load hexameric MCM, the motor of the eukaryotic replicative helicase, into double hexamers at replication origins. Here we show that Cdt1 interacts with MCM ...ORC, Cdc6 and Cdt1 act together to load hexameric MCM, the motor of the eukaryotic replicative helicase, into double hexamers at replication origins. Here we show that Cdt1 interacts with MCM subunits Mcm2, 4 and 6, which both destabilizes the Mcm2-5 interface and inhibits MCM ATPase activity. Using X-ray crystallography, we show that Cdt1 contains two winged-helix domains in the C-terminal half of the protein and a catalytically inactive dioxygenase-related N-terminal domain, which is important for MCM loading, but not for subsequent replication. We used these structures together with single-particle electron microscopy to generate three-dimensional models of MCM complexes. These show that Cdt1 stabilizes MCM in a left-handed spiral open at the Mcm2-5 gate. We propose that Cdt1 acts as a brace, holding MCM open for DNA entry and bound to ATP until ORC-Cdc6 triggers ATP hydrolysis by MCM, promoting both Cdt1 ejection and MCM ring closure. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3679.map.gz emd_3679.map.gz | 7.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3679-v30.xml emd-3679-v30.xml emd-3679.xml emd-3679.xml | 7.9 KB 7.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3679_fsc.xml emd_3679_fsc.xml | 5.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_3679.png emd_3679.png | 460.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3679 http://ftp.pdbj.org/pub/emdb/structures/EMD-3679 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3679 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3679 | HTTPS FTP |
-Related structure data
| Related structure data |  3680C  3681C  5me9C  5meaC  5mebC  5mecC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3679.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3679.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 2.7 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Full-length yeast Cdt1-MCM complex in apo state.
| Entire | Name: Full-length yeast Cdt1-MCM complex in apo state. |
|---|---|
| Components |
|
-Supramolecule #1: Full-length yeast Cdt1-MCM complex in apo state.
| Supramolecule | Name: Full-length yeast Cdt1-MCM complex in apo state. / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
| Recombinant expression | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Staining | Type: NEGATIVE / Material: Uranyl formate |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2100 |
|---|---|
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Average electron dose: 20.0 e/Å2 |
 Movie
Movie Controller
Controller