+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of RCD-1 pore from Neurospora crassa | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Pyroptosis / Gasdermnin / Pore / Pyroptosis / Gasdermnin / Pore /  Allorecognition / Allorecognition /  IMMUNE SYSTEM IMMUNE SYSTEM | |||||||||
| Function / homology | wide pore channel activity /  programmed cell death / protein heterodimerization activity / programmed cell death / protein heterodimerization activity /  plasma membrane / plasma membrane /  cytoplasm / Gasdermin-like protein rcd-1-2 / Gasdermin-like protein rcd-1-1 cytoplasm / Gasdermin-like protein rcd-1-2 / Gasdermin-like protein rcd-1-1 Function and homology information Function and homology information | |||||||||
| Biological species |   Neurospora crassa (fungus) Neurospora crassa (fungus) | |||||||||
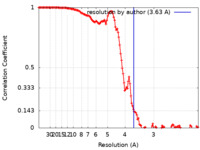
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.63 Å cryo EM / Resolution: 3.63 Å | |||||||||
 Authors Authors | Hou YJ / Sun Q / Li Y / Ding J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2024 Journal: Science / Year: 2024Title: Cleavage-independent activation of ancient eukaryotic gasdermins and structural mechanisms. Authors: Yueyue Li / Yanjie Hou / Qi Sun / Huan Zeng / Fanyi Meng / Xiang Tian / Qun He / Feng Shao / Jingjin Ding /  Abstract: Gasdermins (GSDMs) are pore-forming proteins that execute pyroptosis for immune defense. GSDMs are two-domain proteins, activated by proteolytic removal of the inhibitory domain. Here we report two ...Gasdermins (GSDMs) are pore-forming proteins that execute pyroptosis for immune defense. GSDMs are two-domain proteins, activated by proteolytic removal of the inhibitory domain. Here we report two types of cleavage-independent GSDM activation. First, GSDM, a pore-forming-domain-only protein from the basal metazoan , is a disulfides-linked autoinhibited dimer, activated by reduction of the disulfides. Cryo-electron microscopy (cryo-EM) structure illustrates assembly mechanism for the 44-mer GSDM pore. Second, RCD-1-1/RCD-1-2, encoded by polymorphic in filamentous fungus , are also pore-forming-domain-only GSDMs. RCD-1-1 and RCD-1-2, when encountering each other, form pores and cause pyroptosis, underlying allorecognition in . Cryo-EM structure reveals a pore of 11 RCD-1-1/RCD-1-2 heterodimers and heterodimerization-triggered pore assembly mechanism. This study shows mechanistic diversities in GSDM activation and indicates versatile functions of GSDMs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36734.map.gz emd_36734.map.gz | 197.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36734-v30.xml emd-36734-v30.xml emd-36734.xml emd-36734.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36734_fsc.xml emd_36734_fsc.xml | 13.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_36734.png emd_36734.png | 178.8 KB | ||
| Filedesc metadata |  emd-36734.cif.gz emd-36734.cif.gz | 5.8 KB | ||
| Others |  emd_36734_half_map_1.map.gz emd_36734_half_map_1.map.gz emd_36734_half_map_2.map.gz emd_36734_half_map_2.map.gz | 193.5 MB 193.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36734 http://ftp.pdbj.org/pub/emdb/structures/EMD-36734 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36734 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36734 | HTTPS FTP |
-Related structure data
| Related structure data |  8jyzMC  8jyvC  8jywC  8jyxC  8jyyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36734.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36734.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36734_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36734_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : RCD-1 pore from Neurospora crassa
| Entire | Name: RCD-1 pore from Neurospora crassa |
|---|---|
| Components |
|
-Supramolecule #1: RCD-1 pore from Neurospora crassa
| Supramolecule | Name: RCD-1 pore from Neurospora crassa / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 620 KDa |
-Macromolecule #1: Gasdermin-like protein rcd-1-1
| Macromolecule | Name: Gasdermin-like protein rcd-1-1 / type: protein_or_peptide / ID: 1 / Number of copies: 11 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 29.218693 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: SGRPMDKCWF TLDNAHYPPP SLDSMRSGHP ISPASLGHLI PSLAHLDQII NAKAIEPFPA TMDIHGPTII EDFKWDHSHE YSLSLGGKV PIPLAPAGVP FVDLNVGLGG AFSRSVANYW EFDRLERYIM QPTRSYVQKC IERDEVKRWI AKNKSMMMMG R WEVYMITG ...String: SGRPMDKCWF TLDNAHYPPP SLDSMRSGHP ISPASLGHLI PSLAHLDQII NAKAIEPFPA TMDIHGPTII EDFKWDHSHE YSLSLGGKV PIPLAPAGVP FVDLNVGLGG AFSRSVANYW EFDRLERYIM QPTRSYVQKC IERDEVKRWI AKNKSMMMMG R WEVYMITG IIVARGGGRK KKEKTTGKEF SVEVTVEVPL IVEAGPGGKR NTARQKTWGT SQTGDFVWAV RLAKITKSGL HS DWKMETV FGKTSSFRGQ KAIF UniProtKB: Gasdermin-like protein rcd-1-1 |
-Macromolecule #2: Gasdermin-like protein rcd-1-2
| Macromolecule | Name: Gasdermin-like protein rcd-1-2 / type: protein_or_peptide / ID: 2 / Number of copies: 11 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Neurospora crassa (fungus) Neurospora crassa (fungus) |
| Molecular weight | Theoretical: 27.630418 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: SGRPMDNEEW FPLKQTHYPP PTIPSMKTGH PTGPISIGHI IPDLRHLDNV INCKGFEPFP PNMDVFTAHY EQCHFGDHLN SEFVVQAKA AAPIENIVPG VDVTGSAGLH HTNITSDRWE YDSVVEYAVY PTRQYIDRLL ESKEVRQYIQ KSKKLLGGWC V YMVTGIMV ...String: SGRPMDNEEW FPLKQTHYPP PTIPSMKTGH PTGPISIGHI IPDLRHLDNV INCKGFEPFP PNMDVFTAHY EQCHFGDHLN SEFVVQAKA AAPIENIVPG VDVTGSAGLH HTNITSDRWE YDSVVEYAVY PTRQYIDRLL ESKEVRQYIQ KSKKLLGGWC V YMVTGIMV ARGGGRNVTS EEKGAGVSGN VGFQVPGIGE FAPEVGWDTK TKTKVNAHHT TDFVCAIRLV KIAKSGLRSS WT MKKVTRE F UniProtKB: Gasdermin-like protein rcd-1-2 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE OXIDE / Pretreatment - Type: GLOW DISCHARGE | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 56.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X