[English] 日本語
 Yorodumi
Yorodumi- EMDB-36342: Cryo-EM structure of the beta2AR-mBRIL/1b3 Fab/Glue complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the beta2AR-mBRIL/1b3 Fab/Glue complex with a partial agonist | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  complex / complex /  chimera / chimera /  GPCR / GPCR /  membrane protein membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationdesensitization of G protein-coupled receptor signaling pathway by arrestin / beta2-adrenergic receptor activity / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of mini excitatory postsynaptic potential / positive regulation of cAMP-dependent protein kinase activity /  norepinephrine binding / norepinephrine binding /  Adrenoceptors / Adrenoceptors /  heat generation / positive regulation of autophagosome maturation / positive regulation of AMPA receptor activity ...desensitization of G protein-coupled receptor signaling pathway by arrestin / beta2-adrenergic receptor activity / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of mini excitatory postsynaptic potential / positive regulation of cAMP-dependent protein kinase activity / heat generation / positive regulation of autophagosome maturation / positive regulation of AMPA receptor activity ...desensitization of G protein-coupled receptor signaling pathway by arrestin / beta2-adrenergic receptor activity / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of mini excitatory postsynaptic potential / positive regulation of cAMP-dependent protein kinase activity /  norepinephrine binding / norepinephrine binding /  Adrenoceptors / Adrenoceptors /  heat generation / positive regulation of autophagosome maturation / positive regulation of AMPA receptor activity / activation of transmembrane receptor protein tyrosine kinase activity / negative regulation of smooth muscle contraction / positive regulation of lipophagy / response to psychosocial stress / negative regulation of multicellular organism growth / endosome to lysosome transport / adrenergic receptor signaling pathway / diet induced thermogenesis / neuronal dense core vesicle / positive regulation of protein kinase A signaling / heat generation / positive regulation of autophagosome maturation / positive regulation of AMPA receptor activity / activation of transmembrane receptor protein tyrosine kinase activity / negative regulation of smooth muscle contraction / positive regulation of lipophagy / response to psychosocial stress / negative regulation of multicellular organism growth / endosome to lysosome transport / adrenergic receptor signaling pathway / diet induced thermogenesis / neuronal dense core vesicle / positive regulation of protein kinase A signaling /  adenylate cyclase binding / smooth muscle contraction / potassium channel regulator activity / positive regulation of bone mineralization / adenylate cyclase-activating adrenergic receptor signaling pathway / brown fat cell differentiation / regulation of sodium ion transport / adenylate cyclase binding / smooth muscle contraction / potassium channel regulator activity / positive regulation of bone mineralization / adenylate cyclase-activating adrenergic receptor signaling pathway / brown fat cell differentiation / regulation of sodium ion transport /  bone resorption / activation of adenylate cyclase activity / response to cold / bone resorption / activation of adenylate cyclase activity / response to cold /  receptor-mediated endocytosis / clathrin-coated endocytic vesicle membrane / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / positive regulation of protein serine/threonine kinase activity / cellular response to amyloid-beta / Cargo recognition for clathrin-mediated endocytosis / receptor-mediated endocytosis / clathrin-coated endocytic vesicle membrane / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / positive regulation of protein serine/threonine kinase activity / cellular response to amyloid-beta / Cargo recognition for clathrin-mediated endocytosis /  Clathrin-mediated endocytosis / positive regulation of cold-induced thermogenesis / Clathrin-mediated endocytosis / positive regulation of cold-induced thermogenesis /  amyloid-beta binding / G alpha (s) signalling events / positive regulation of MAPK cascade / transcription by RNA polymerase II / amyloid-beta binding / G alpha (s) signalling events / positive regulation of MAPK cascade / transcription by RNA polymerase II /  electron transfer activity / electron transfer activity /  lysosome / lysosome /  periplasmic space / cell surface receptor signaling pathway / periplasmic space / cell surface receptor signaling pathway /  receptor complex / receptor complex /  early endosome / endosome membrane / Ub-specific processing proteases / early endosome / endosome membrane / Ub-specific processing proteases /  endosome / iron ion binding / apical plasma membrane / endosome / iron ion binding / apical plasma membrane /  heme binding / protein-containing complex binding / heme binding / protein-containing complex binding /  Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II /  membrane / identical protein binding / membrane / identical protein binding /  nucleus / nucleus /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | He BB / Zhong YX / Guo Q / Tao YY | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: A method for structure determination of GPCRs in various states. Authors: Qiong Guo / Binbin He / Yixuan Zhong / Haizhan Jiao / Yinhang Ren / Qinggong Wang / Qiangqiang Ge / Yongxiang Gao / Xiangyu Liu / Yang Du / Hongli Hu / Yuyong Tao /  Abstract: G-protein-coupled receptors (GPCRs) are a class of integral membrane proteins that detect environmental cues and trigger cellular responses. Deciphering the functional states of GPCRs induced by ...G-protein-coupled receptors (GPCRs) are a class of integral membrane proteins that detect environmental cues and trigger cellular responses. Deciphering the functional states of GPCRs induced by various ligands has been one of the primary goals in the field. Here we developed an effective universal method for GPCR cryo-electron microscopy structure determination without the need to prepare GPCR-signaling protein complexes. Using this method, we successfully solved the structures of the β-adrenergic receptor (βAR) bound to antagonistic and agonistic ligands and the adhesion GPCR ADGRL3 in the apo state. For βAR, an intermediate state stabilized by the partial agonist was captured. For ADGRL3, the structure revealed that inactive ADGRL3 adopts a compact fold and that large unusual conformational changes on both the extracellular and intracellular sides are required for activation of adhesion GPCRs. We anticipate that this method will open a new avenue for understanding GPCR structure‒function relationships and drug development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36342.map.gz emd_36342.map.gz | 79 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36342-v30.xml emd-36342-v30.xml emd-36342.xml emd-36342.xml | 14.3 KB 14.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_36342_fsc.xml emd_36342_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_36342.png emd_36342.png | 97.3 KB | ||
| Filedesc metadata |  emd-36342.cif.gz emd-36342.cif.gz | 5.7 KB | ||
| Others |  emd_36342_half_map_1.map.gz emd_36342_half_map_1.map.gz emd_36342_half_map_2.map.gz emd_36342_half_map_2.map.gz | 77.7 MB 77.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36342 http://ftp.pdbj.org/pub/emdb/structures/EMD-36342 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36342 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36342 | HTTPS FTP |
-Related structure data
| Related structure data |  8jj8MC  8j7eC  8jjlC  8jjoC  8jmtC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36342.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36342.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36342_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36342_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of the beta2AR-mBRIL/1b3 Fab/Glue complex with ...
| Entire | Name: Cryo-EM structure of the beta2AR-mBRIL/1b3 Fab/Glue complex with a partial agonist |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the beta2AR-mBRIL/1b3 Fab/Glue complex with ...
| Supramolecule | Name: Cryo-EM structure of the beta2AR-mBRIL/1b3 Fab/Glue complex with a partial agonist type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Beta-2 adrenergic receptor,Soluble cytochrome b562
| Macromolecule | Name: Beta-2 adrenergic receptor,Soluble cytochrome b562 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.594102 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKDEVWVV GMGIVMSLIV LAIVFGNVLV ITAIAKFERL QTVTNYFITS LACADLVMGL AVVPFGAAH ILMKMWTFGN FWCEFWTSID VLCVTASIET LCVIAVDRYF AITSPFKYQS LLTKNKARVI ILMVWIVSGL T SFLPIQMH ...String: MKTIIALSYI FCLVFADYKD DDDKDEVWVV GMGIVMSLIV LAIVFGNVLV ITAIAKFERL QTVTNYFITS LACADLVMGL AVVPFGAAH ILMKMWTFGN FWCEFWTSID VLCVTASIET LCVIAVDRYF AITSPFKYQS LLTKNKARVI ILMVWIVSGL T SFLPIQMH WYRATHQEAI NCYAEETCCD FFTNQAYAIA SSIVSFYVPL VIMVFVYSRV FQEAKRQLAD LEDNWETLND NL KVIEKAD NAAQVKDALT KMRAAALDAQ KASGSGSPEM KDFRHGFDIL VGQIDDALKL ANEGKVKEAQ AAAEQLKTTR NAY IQKYLK FCLKEHKALK TLGIIMGTFT LCWLPFFIVN IVHVIQDNLI RKEVYILLNW IGYVNSGFNP LIYSRSPDFR IAFQ ELLKI AALKEKIAAL KEKIAALKEA EEKRASRLEE ELRRRLTEGS HHHHHHHH UniProtKB:  Beta-2 adrenergic receptor, Soluble cytochrome b562, Beta-2 adrenergic receptor, Soluble cytochrome b562,  Beta-2 adrenergic receptor Beta-2 adrenergic receptor |
-Macromolecule #2: ~{N}-[5-[(1~{R})-2-[[(2~{R})-1-(4-methoxyphenyl)propan-2-yl]amino...
| Macromolecule | Name: ~{N}-[5-[(1~{R})-2-[[(2~{R})-1-(4-methoxyphenyl)propan-2-yl]amino]-1-oxidanyl-ethyl]-2-oxidanyl-phenyl]methanamide type: ligand / ID: 2 / Number of copies: 1 / Formula: H98 |
|---|---|
| Molecular weight | Theoretical: 344.405 Da |
| Chemical component information | 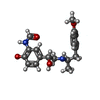 ChemComp-H98: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Material: NICKEL/TITANIUM |
| Vitrification | Cryogen name: NITROGEN |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z
Z Y
Y X
X


















