+ Open data
Open data
- Basic information
Basic information
| Entry | 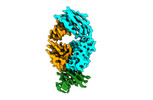 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Tocilizumab Fab/IL-6R complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  antibody / IL-6R / antibody / IL-6R /  structural protein / structural protein /  IMMUNE SYSTEM IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology information ciliary neurotrophic factor binding / ciliary neurotrophic factor binding /  interleukin-6 receptor activity / interleukin-6 receptor activity /  interleukin-6 binding / hepatic immune response / interleukin-6 binding / hepatic immune response /  interleukin-11 receptor activity / interleukin-11 receptor activity /  interleukin-11 binding / interleukin-11 binding /  ciliary neurotrophic factor receptor complex / ciliary neurotrophic factor-mediated signaling pathway / ciliary neurotrophic factor receptor complex / ciliary neurotrophic factor-mediated signaling pathway /  interleukin-6 receptor complex / negative regulation of collagen biosynthetic process ... interleukin-6 receptor complex / negative regulation of collagen biosynthetic process ... ciliary neurotrophic factor binding / ciliary neurotrophic factor binding /  interleukin-6 receptor activity / interleukin-6 receptor activity /  interleukin-6 binding / hepatic immune response / interleukin-6 binding / hepatic immune response /  interleukin-11 receptor activity / interleukin-11 receptor activity /  interleukin-11 binding / interleukin-11 binding /  ciliary neurotrophic factor receptor complex / ciliary neurotrophic factor-mediated signaling pathway / ciliary neurotrophic factor receptor complex / ciliary neurotrophic factor-mediated signaling pathway /  interleukin-6 receptor complex / negative regulation of collagen biosynthetic process / positive regulation of activation of Janus kinase activity / T-helper 17 cell lineage commitment / positive regulation of glomerular mesangial cell proliferation / endocrine pancreas development / negative regulation of interleukin-8 production / vascular endothelial growth factor production / positive regulation of leukocyte chemotaxis / neutrophil mediated immunity / interleukin-6 receptor complex / negative regulation of collagen biosynthetic process / positive regulation of activation of Janus kinase activity / T-helper 17 cell lineage commitment / positive regulation of glomerular mesangial cell proliferation / endocrine pancreas development / negative regulation of interleukin-8 production / vascular endothelial growth factor production / positive regulation of leukocyte chemotaxis / neutrophil mediated immunity /  cytokine receptor activity / Interleukin-6 signaling / interleukin-6-mediated signaling pathway / MAPK3 (ERK1) activation / MAPK1 (ERK2) activation / monocyte chemotaxis / positive regulation of osteoblast differentiation / positive regulation of chemokine production / extrinsic apoptotic signaling pathway / positive regulation of tyrosine phosphorylation of STAT protein / response to cytokine / acute-phase response / positive regulation of smooth muscle cell proliferation / cytokine-mediated signaling pathway / positive regulation of interleukin-6 production / Transcriptional regulation of granulopoiesis / positive regulation of peptidyl-tyrosine phosphorylation / Interleukin-4 and Interleukin-13 signaling / defense response to Gram-negative bacterium / Potential therapeutics for SARS / positive regulation of MAPK cascade / cytokine receptor activity / Interleukin-6 signaling / interleukin-6-mediated signaling pathway / MAPK3 (ERK1) activation / MAPK1 (ERK2) activation / monocyte chemotaxis / positive regulation of osteoblast differentiation / positive regulation of chemokine production / extrinsic apoptotic signaling pathway / positive regulation of tyrosine phosphorylation of STAT protein / response to cytokine / acute-phase response / positive regulation of smooth muscle cell proliferation / cytokine-mediated signaling pathway / positive regulation of interleukin-6 production / Transcriptional regulation of granulopoiesis / positive regulation of peptidyl-tyrosine phosphorylation / Interleukin-4 and Interleukin-13 signaling / defense response to Gram-negative bacterium / Potential therapeutics for SARS / positive regulation of MAPK cascade /  receptor complex / defense response to Gram-positive bacterium / apical plasma membrane / external side of plasma membrane / positive regulation of cell population proliferation / receptor complex / defense response to Gram-positive bacterium / apical plasma membrane / external side of plasma membrane / positive regulation of cell population proliferation /  enzyme binding / protein homodimerization activity / enzyme binding / protein homodimerization activity /  extracellular space / extracellular region / extracellular space / extracellular region /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.3 Å cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | She J / Chen L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structures of tocilizumab and sarilumab Fabs in complex with IL-6R Authors: She J / Wang MX | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36003.map.gz emd_36003.map.gz | 203.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36003-v30.xml emd-36003-v30.xml emd-36003.xml emd-36003.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36003.png emd_36003.png | 42.3 KB | ||
| Filedesc metadata |  emd-36003.cif.gz emd-36003.cif.gz | 5.7 KB | ||
| Others |  emd_36003_half_map_1.map.gz emd_36003_half_map_1.map.gz emd_36003_half_map_2.map.gz emd_36003_half_map_2.map.gz | 200.3 MB 200.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36003 http://ftp.pdbj.org/pub/emdb/structures/EMD-36003 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36003 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36003 | HTTPS FTP |
-Related structure data
| Related structure data |  8j6fMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36003.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36003.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36003_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36003_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : the tocilizumab Fab/IL-6R complex
| Entire | Name: the tocilizumab Fab/IL-6R complex |
|---|---|
| Components |
|
-Supramolecule #1: the tocilizumab Fab/IL-6R complex
| Supramolecule | Name: the tocilizumab Fab/IL-6R complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Heavy chain of Tocilizumab Fab
| Macromolecule | Name: Heavy chain of Tocilizumab Fab / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.12508 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLQESGPG LVRPSQTLSL TCTVSGYSIT SDHAWSWVRQ PPGRGLEWIG YISYSGITTY NPSLKSRVTM LRDTSKNQFS LRLSSVTAA DTAVYYCARS LARTTAMDYW GQGSLVTVSS ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS W NSGALTSG ...String: QVQLQESGPG LVRPSQTLSL TCTVSGYSIT SDHAWSWVRQ PPGRGLEWIG YISYSGITTY NPSLKSRVTM LRDTSKNQFS LRLSSVTAA DTAVYYCARS LARTTAMDYW GQGSLVTVSS ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS W NSGALTSG VHTFPAVLQS SGLYSLSSVV TVPSSSLGTQ TYICNVNHKP SNTKVDKKVE PKSCDKTH |
-Macromolecule #2: Light chain of Tocilizumab Fab
| Macromolecule | Name: Light chain of Tocilizumab Fab / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.527078 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DIQMTQSPSS LSASVGDRVT ITCRASQDIS SYLNWYQQKP GKAPKLLIYY TSRLHSGVPS RFSGSGSGTD FTFTISSLQP EDIATYYCQ QGNTLPYTFG QGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS ...String: DIQMTQSPSS LSASVGDRVT ITCRASQDIS SYLNWYQQKP GKAPKLLIYY TSRLHSGVPS RFSGSGSGTD FTFTISSLQP EDIATYYCQ QGNTLPYTFG QGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS KDSTYSLSST LTLSKADYEK HKVYACEVTH QGLSSPVTKS FNRGEC |
-Macromolecule #3: Interleukin-6 receptor subunit alpha
| Macromolecule | Name: Interleukin-6 receptor subunit alpha / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.283441 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MLAVGCALLA ALLAAPGAAL APRRCPAQEV ARGVLTSLPG DSVTLTCPGV EPEDNATVHW VLRKPAAGSH PSRWAGMGRR LLLRSVQLH DSGNYSCYRA GRPAGTVHLL VDVPPEEPQL SCFRKSPLSN VVCEWGPRST PSLTTKAVLL VRKFQNSPAE D FQEPCQYS ...String: MLAVGCALLA ALLAAPGAAL APRRCPAQEV ARGVLTSLPG DSVTLTCPGV EPEDNATVHW VLRKPAAGSH PSRWAGMGRR LLLRSVQLH DSGNYSCYRA GRPAGTVHLL VDVPPEEPQL SCFRKSPLSN VVCEWGPRST PSLTTKAVLL VRKFQNSPAE D FQEPCQYS QESQKFSCQL AVPEGDSSFY IVSMCVASSV GSKFSKTQTF QGCGILQPDP PANITVTAVA RNPRWLSVTW QD PHSWNSS FYRLRFELRY RAERSKTFTT WMVKDLQHHC VIHDAWSGLR HVVQLRAQEE FGQGEWSEWS PEAMGTPWTE SRS PPAENE VSTPMQALTT NKDDDNILFR DSANATSLPG SRRRGSCGL UniProtKB:  Interleukin-6 receptor subunit alpha Interleukin-6 receptor subunit alpha |
-Macromolecule #4: IL6R-D2 peptide
| Macromolecule | Name: IL6R-D2 peptide / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.148355 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: FRKSPLSNVV UniProtKB:  Interleukin-6 receptor subunit alpha Interleukin-6 receptor subunit alpha |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 1 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.7 µm / Nominal defocus min: 1.4000000000000001 µm Bright-field microscopy / Nominal defocus max: 2.7 µm / Nominal defocus min: 1.4000000000000001 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 55.0 e/Å2 |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 351599 |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X

















