[English] 日本語
 Yorodumi
Yorodumi- EMDB-33147: Cryo-EM structures of human mitochondrial NAD(P)+-dependent malic... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structures of human mitochondrial NAD(P)+-dependent malic enzyme in a ternary complex with NAD+ and allosteric inhibitor MDSA | |||||||||
 Map data Map data | ME2-MDSA Full Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Human mitochondrial NAD(P)+-dependent malic enzyme (ME2) /  inhibitor / inhibitor /  HYDROLASE HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology information malate dehydrogenase (oxaloacetate-decarboxylating) / malate dehydrogenase (decarboxylating) (NAD+) activity / regulation of NADP metabolic process / malate dehydrogenase (decarboxylating) (NADP+) activity / malate dehydrogenase (oxaloacetate-decarboxylating) / malate dehydrogenase (decarboxylating) (NAD+) activity / regulation of NADP metabolic process / malate dehydrogenase (decarboxylating) (NADP+) activity /  malic enzyme activity / malic enzyme activity /  oxaloacetate decarboxylase activity / Citric acid cycle (TCA cycle) / malate metabolic process / pyruvate metabolic process / NAD binding ... oxaloacetate decarboxylase activity / Citric acid cycle (TCA cycle) / malate metabolic process / pyruvate metabolic process / NAD binding ... malate dehydrogenase (oxaloacetate-decarboxylating) / malate dehydrogenase (decarboxylating) (NAD+) activity / regulation of NADP metabolic process / malate dehydrogenase (decarboxylating) (NADP+) activity / malate dehydrogenase (oxaloacetate-decarboxylating) / malate dehydrogenase (decarboxylating) (NAD+) activity / regulation of NADP metabolic process / malate dehydrogenase (decarboxylating) (NADP+) activity /  malic enzyme activity / malic enzyme activity /  oxaloacetate decarboxylase activity / Citric acid cycle (TCA cycle) / malate metabolic process / pyruvate metabolic process / NAD binding / oxaloacetate decarboxylase activity / Citric acid cycle (TCA cycle) / malate metabolic process / pyruvate metabolic process / NAD binding /  electron transfer activity / electron transfer activity /  mitochondrial matrix / intracellular membrane-bounded organelle / mitochondrial matrix / intracellular membrane-bounded organelle /  mitochondrion / mitochondrion /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.84 Å cryo EM / Resolution: 2.84 Å | |||||||||
 Authors Authors | Wang CH / Hsieh JT / Ho MC / Hung HC | |||||||||
| Funding support |  Taiwan, 2 items Taiwan, 2 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2023 Journal: Commun Biol / Year: 2023Title: Suppression of the human malic enzyme 2 modifies energy metabolism and inhibits cellular respiration Authors: Hsieh JY / Chen KC / Wang CH / Liu GY / Ye JA / Chou YT / Lin YC / Lyu CJ / Chang RY / Liu YL / Li YH / Lee MR / Ho MC / Hung HC | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33147.map.gz emd_33147.map.gz | 203.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33147-v30.xml emd-33147-v30.xml emd-33147.xml emd-33147.xml | 15 KB 15 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33147_fsc.xml emd_33147_fsc.xml | 13.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_33147.png emd_33147.png | 198.5 KB | ||
| Others |  emd_33147_half_map_1.map.gz emd_33147_half_map_1.map.gz emd_33147_half_map_2.map.gz emd_33147_half_map_2.map.gz | 199.8 MB 199.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33147 http://ftp.pdbj.org/pub/emdb/structures/EMD-33147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33147 | HTTPS FTP |
-Related structure data
| Related structure data |  7xdgMC  7xdeC  7xdfC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33147.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33147.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ME2-MDSA Full Map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||
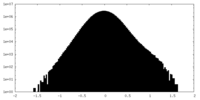
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: ME2-MDSA Half Map 1
| File | emd_33147_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ME2-MDSA Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
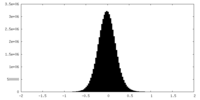
| Density Histograms |
-Half map: ME2-MDSA Half Map 2
| File | emd_33147_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ME2-MDSA Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
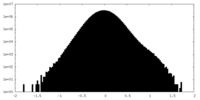
| Density Histograms |
- Sample components
Sample components
-Entire : human mitochondrial NAD(P)+-dependent malic enzyme in apo form
| Entire | Name: human mitochondrial NAD(P)+-dependent malic enzyme in apo form |
|---|---|
| Components |
|
-Supramolecule #1: human mitochondrial NAD(P)+-dependent malic enzyme in apo form
| Supramolecule | Name: human mitochondrial NAD(P)+-dependent malic enzyme in apo form type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: NAD-dependent malic enzyme, mitochondrial
| Macromolecule | Name: NAD-dependent malic enzyme, mitochondrial / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO EC number:  malate dehydrogenase (oxaloacetate-decarboxylating) malate dehydrogenase (oxaloacetate-decarboxylating) |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 65.521434 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: MLSRLRVVST TCTLACRHLH IKEKGKPLML NPRTNKGMAF TLQERQMLGL QGLLPPKIET QDIQALRFHR NLKKMTSPLE KYIYIMGIQ ERNEKLFYRI LQDDIESLMP IVYTPTVGLA CSQYGHIFRR PKGLFISISD RGHVRSIVDN WPENHVKAVV V TDGERILG ...String: MLSRLRVVST TCTLACRHLH IKEKGKPLML NPRTNKGMAF TLQERQMLGL QGLLPPKIET QDIQALRFHR NLKKMTSPLE KYIYIMGIQ ERNEKLFYRI LQDDIESLMP IVYTPTVGLA CSQYGHIFRR PKGLFISISD RGHVRSIVDN WPENHVKAVV V TDGERILG LGDLGVYGMG IPVGKLCLYT ACAGIRPDRC LPVCIDVGTD NIALLKDPFY MGLYQKRDRT QQYDDLIDEF MK AITDRYG RNTLIQFEDF GNHNAFRFLR KYREKYCTFN DDIQGTAAVA LAGLLAAQKV ISKPISEHKI LFLGAGEAAL GIA NLIVMS MVENGLSEQE AQKKIWMFDK YGLLVKGRKA KIDSYQEPFT HSAPESIPDT FEDAVNILKP STIIGVAGAG RLFT PDVIR AMASINERPV IFALSNPTAQ AECTAEEAYT LTEGRCLFAS GSPFGPVKLT DGRVFTPGQG NNVYIFPGVA LAVIL CNTR HISDSVFLEA AKALTSQLTD EELAQGRLYP PLANIQEVSI NIAIKVTEYL YANKMAFRYP EPEDKAKYVK ERTWRS EYD SLLPDVYEWP ESASSPPVIT E |
-Macromolecule #2: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
| Macromolecule | Name: NICOTINAMIDE-ADENINE-DINUCLEOTIDE / type: ligand / ID: 2 / Number of copies: 8 / Formula: NAD |
|---|---|
| Molecular weight | Theoretical: 663.425 Da |
| Chemical component information |  ChemComp-NAD: |
-Macromolecule #3: 5-[(3-carboxy-4-oxidanyl-phenyl)methyl]-2-oxidanyl-benzoic acid
| Macromolecule | Name: 5-[(3-carboxy-4-oxidanyl-phenyl)methyl]-2-oxidanyl-benzoic acid type: ligand / ID: 3 / Number of copies: 4 / Formula: D5S |
|---|---|
| Molecular weight | Theoretical: 288.252 Da |
| Chemical component information |  ChemComp-D5S: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.3000000000000003 µm / Nominal defocus min: 0.5 µm Bright-field microscopy / Nominal defocus max: 3.3000000000000003 µm / Nominal defocus min: 0.5 µm |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z
Z Y
Y X
X