[English] 日本語
 Yorodumi
Yorodumi- EMDB-31490: Human pannexin-1 showing a conformational change in the N-termina... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31490 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human pannexin-1 showing a conformational change in the N-terminal domain and blocked pore | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationElectric Transmission Across Gap Junctions /  leak channel activity / positive regulation of interleukin-1 alpha production / bleb / wide pore channel activity / gap junction channel activity / leak channel activity / positive regulation of interleukin-1 alpha production / bleb / wide pore channel activity / gap junction channel activity /  gap junction / positive regulation of macrophage cytokine production / gap junction / positive regulation of macrophage cytokine production /  oogenesis / response to ATP ...Electric Transmission Across Gap Junctions / oogenesis / response to ATP ...Electric Transmission Across Gap Junctions /  leak channel activity / positive regulation of interleukin-1 alpha production / bleb / wide pore channel activity / gap junction channel activity / leak channel activity / positive regulation of interleukin-1 alpha production / bleb / wide pore channel activity / gap junction channel activity /  gap junction / positive regulation of macrophage cytokine production / gap junction / positive regulation of macrophage cytokine production /  oogenesis / response to ATP / monoatomic cation transport / The NLRP3 inflammasome / positive regulation of interleukin-1 beta production / response to ischemia / oogenesis / response to ATP / monoatomic cation transport / The NLRP3 inflammasome / positive regulation of interleukin-1 beta production / response to ischemia /  calcium channel activity / calcium ion transport / calcium channel activity / calcium ion transport /  actin filament binding / cell-cell signaling / actin filament binding / cell-cell signaling /  scaffold protein binding / scaffold protein binding /  protease binding / transmembrane transporter binding / protease binding / transmembrane transporter binding /  signaling receptor binding / endoplasmic reticulum membrane / structural molecule activity / signaling receptor binding / endoplasmic reticulum membrane / structural molecule activity /  endoplasmic reticulum / protein-containing complex / endoplasmic reticulum / protein-containing complex /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
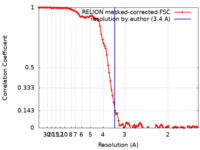
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.4 Å cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Kuzuya M / Hirano H / Hayashida K / Watanabe M / Kobayashi K / Tani K / Fujiyoshi Y / Oshima A | |||||||||
 Citation Citation |  Journal: Sci Signal / Year: 2022 Journal: Sci Signal / Year: 2022Title: Structures of human pannexin-1 in nanodiscs reveal gating mediated by dynamic movement of the N terminus and phospholipids. Authors: Maki Kuzuya / Hidemi Hirano / Kenichi Hayashida / Masakatsu Watanabe / Kazumi Kobayashi / Tohru Terada / Md Iqbal Mahmood / Florence Tama / Kazutoshi Tani / Yoshinori Fujiyoshi / Atsunori Oshima /  Abstract: Pannexin (PANX) family proteins form large-pore channels that mediate purinergic signaling. We analyzed the cryo-EM structures of human PANX1 in lipid nanodiscs to elucidate the gating mechanism and ...Pannexin (PANX) family proteins form large-pore channels that mediate purinergic signaling. We analyzed the cryo-EM structures of human PANX1 in lipid nanodiscs to elucidate the gating mechanism and its regulation by the amino terminus in phospholipids. The wild-type channel has an amino-terminal funnel in the pore, but in the presence of the inhibitor probenecid, a cytoplasmically oriented amino terminus and phospholipids obstruct the pore. Functional analysis using whole-cell patch-clamp and oocyte voltage clamp showed that PANX1 lacking the amino terminus did not open and had a dominant negative effect on channel activity, thus confirming that the amino-terminal domain played an essential role in channel opening. These observations suggest that dynamic conformational changes in the amino terminus of human PANX1 are associated with lipid movement in and out of the pore. Moreover, the data provide insight into the gating mechanism of PANX1 and, more broadly, other large-pore channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31490.map.gz emd_31490.map.gz | 12.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31490-v30.xml emd-31490-v30.xml emd-31490.xml emd-31490.xml | 16.8 KB 16.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_31490_fsc.xml emd_31490_fsc.xml | 10 KB | Display |  FSC data file FSC data file |
| Images |  emd_31490.png emd_31490.png | 231.1 KB | ||
| Masks |  emd_31490_msk_1.map emd_31490_msk_1.map | 83.7 MB |  Mask map Mask map | |
| Others |  emd_31490_half_map_1.map.gz emd_31490_half_map_1.map.gz emd_31490_half_map_2.map.gz emd_31490_half_map_2.map.gz | 65.5 MB 65.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31490 http://ftp.pdbj.org/pub/emdb/structures/EMD-31490 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31490 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31490 | HTTPS FTP |
-Related structure data
| Related structure data |  7f8nMC  7f8jC  7f8oC  7wsvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10758 (Title: Structure of human pannexin-1 with a blocked pore / Data size: 459.1 EMPIAR-10758 (Title: Structure of human pannexin-1 with a blocked pore / Data size: 459.1 Data #1: human PANX1 in a blocked state [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31490.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31490.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.81 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_31490_msk_1.map emd_31490_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_31490_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_31490_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human pannexin-1
| Entire | Name: human pannexin-1 |
|---|---|
| Components |
|
-Supramolecule #1: human pannexin-1
| Supramolecule | Name: human pannexin-1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Pannexin-1
| Macromolecule | Name: Pannexin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 48.093863 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAIAQLATEY VFSDFLLKEP TEPKFKGLRL ELAVDKMVTC IAVGLPLLLI SLAFAQEISI GTQISCFSPS SFSWRQAAFV DSYCWAAVQ QKNSLQSESG NLPLWLHKFF PYILLLFAIL LYLPPLFWRF AAAPHICSDL KFIMEELDKV YNRAIKAAKS A RDLDMRDG ...String: MAIAQLATEY VFSDFLLKEP TEPKFKGLRL ELAVDKMVTC IAVGLPLLLI SLAFAQEISI GTQISCFSPS SFSWRQAAFV DSYCWAAVQ QKNSLQSESG NLPLWLHKFF PYILLLFAIL LYLPPLFWRF AAAPHICSDL KFIMEELDKV YNRAIKAAKS A RDLDMRDG ACSVPGVTEN LGQSLWEVSE SHFKYPIVEQ YLKTKKNSNN LIIKYISCRL LTLIIILLAC IYLGYYFSLS SL SDEFVCS IKSGILRNDS TVPDQFQCKL IAVGIFQLLS VINLVVYVLL APVVVYTLFV PFRQKTDVLK VYEILPTFDV LHF KSEGYN DLSLYNLFLE ENISEVKSYK CLKVLENIKS SGQGIDPMLL LTNLGMIKMD VVDGKTPMSA EMREEQGNQT AELQ GMNID SETKANNGEK NARQRLLDSS C |
-Macromolecule #2: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
| Macromolecule | Name: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine / type: ligand / ID: 2 / Number of copies: 49 / Formula: LBN |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-LBN: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.0 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Quantifoil R2/2 / Material: MOLYBDENUM / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE | |||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: LEICA KF80 |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 5.0 µm / Calibrated defocus min: 0.5 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm Bright-field microscopy / Cs: 2.7 mm |
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Slit width: 30 eV |
| Sample stage | Specimen holder model: JEOL / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number real images: 1083 / Average exposure time: 2.4 sec. / Average electron dose: 40.0 e/Å2 |
 Movie
Movie Controller
Controller





















 Z
Z Y
Y X
X