[English] 日本語
 Yorodumi
Yorodumi- EMDB-29534: Cryo-EM structure of Stanieria sp. CphA2 in complex with ADPCP an... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Stanieria sp. CphA2 in complex with ADPCP and 4x(beta-Asp-Arg) | |||||||||
 Map data Map data | Locally filtered map of Stanieria sp. CphA2 with ADPCP and 4x(Asp-Arg) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  cyanophycin / CphA2 / cyanophycin / CphA2 /  ligase / ATP-grasp ligase / ATP-grasp | |||||||||
| Function / homology | Carbamoyl-phosphate synthetase large subunit-like, ATP-binding domain / Carbamoyl-phosphate synthase L chain, ATP binding domain / ATP-grasp fold, subdomain 1 / ATP-grasp fold / ATP-grasp fold profile. /  catalytic activity / catalytic activity /  ATP binding / ATP binding /  metal ion binding / RimK domain-containing protein ATP-grasp metal ion binding / RimK domain-containing protein ATP-grasp Function and homology information Function and homology information | |||||||||
| Biological species |  Stanieria sp. (bacteria) / Stanieria sp. NIES-3757 / synthetic construct (others) Stanieria sp. (bacteria) / Stanieria sp. NIES-3757 / synthetic construct (others) | |||||||||
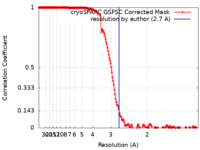
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.7 Å cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Markus LM / Sharon I / Strauss M / Schmeing TM | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Protein Sci / Year: 2023 Journal: Protein Sci / Year: 2023Title: Structure and function of a hexameric cyanophycin synthetase 2. Authors: Linda M D Markus / Itai Sharon / Kim Munro / Marcel Grogg / Donald Hilvert / Mike Strauss / T Martin Schmeing /   Abstract: Cyanophycin is a natural polymer composed of a poly-aspartate backbone with arginine attached to each of the aspartate sidechains. Produced by a wide range of bacteria, which mainly use it as a store ...Cyanophycin is a natural polymer composed of a poly-aspartate backbone with arginine attached to each of the aspartate sidechains. Produced by a wide range of bacteria, which mainly use it as a store of fixed nitrogen, it has many promising industrial applications. Cyanophycin can be synthesized from the amino acids Asp and Arg by the widespread cyanophycin synthetase 1 (CphA1), or from the dipeptide β-Asp-Arg by the cyanobacterial enzyme cyanophycin synthetase 2 (CphA2). CphA2 enzymes display a range of oligomeric states, from dimers to dodecamers. Recently, the crystal structure of a CphA2 dimer was solved but could not be obtained in complex with substrate. Here, we report cryo-EM structures of the hexameric CphA2 from Stanieria sp. at ~2.8 Å resolution, both with and without ATP analog and cyanophycin. The structures show a two-fold symmetrical, trimer-of-dimers hexameric architecture, and substrate-binding interactions that are similar to those of CphA1. Mutagenesis experiments demonstrate the importance of several conserved substrate-binding residues. We also find that a Q416A/R528G double mutation prevents hexamer formation and use this double mutant to show that hexamerization augments the rate of cyanophycin synthesis. Together, these results increase our mechanistic understanding of how an interesting green polymer is biosynthesized. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29534.map.gz emd_29534.map.gz | 22.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29534-v30.xml emd-29534-v30.xml emd-29534.xml emd-29534.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29534_fsc.xml emd_29534_fsc.xml | 16.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_29534.png emd_29534.png | 64.8 KB | ||
| Masks |  emd_29534_msk_1.map emd_29534_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-29534.cif.gz emd-29534.cif.gz | 6.1 KB | ||
| Others |  emd_29534_half_map_1.map.gz emd_29534_half_map_1.map.gz emd_29534_half_map_2.map.gz emd_29534_half_map_2.map.gz | 474.5 MB 474.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29534 http://ftp.pdbj.org/pub/emdb/structures/EMD-29534 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29534 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29534 | HTTPS FTP |
-Related structure data
| Related structure data |  8fxiMC  8fxhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29534.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29534.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally filtered map of Stanieria sp. CphA2 with ADPCP and 4x(Asp-Arg) | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.675 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_29534_msk_1.map emd_29534_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A of Stanieria sp. CphA2 with ADPCP and 4x(Asp-Arg)
| File | emd_29534_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A of Stanieria sp. CphA2 with ADPCP and 4x(Asp-Arg) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B of Stanieria sp. CphA2 with ADPCP and 4x(Asp-Arg)
| File | emd_29534_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B of Stanieria sp. CphA2 with ADPCP and 4x(Asp-Arg) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Stanieria sp. CphA2 in complex with ADPCP and 4x(beta-Asp-Arg)
| Entire | Name: Stanieria sp. CphA2 in complex with ADPCP and 4x(beta-Asp-Arg) |
|---|---|
| Components |
|
-Supramolecule #1: Stanieria sp. CphA2 in complex with ADPCP and 4x(beta-Asp-Arg)
| Supramolecule | Name: Stanieria sp. CphA2 in complex with ADPCP and 4x(beta-Asp-Arg) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Stanieria sp. (bacteria) Stanieria sp. (bacteria) |
-Macromolecule #1: RimK domain-containing protein ATP-grasp
| Macromolecule | Name: RimK domain-containing protein ATP-grasp / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: Stanieria sp. NIES-3757 |
| Molecular weight | Theoretical: 72.886516 KDa |
| Recombinant expression | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Sequence | String: MLTKQAVEPV RINARTTDVF DIFNVKQYVG ANPYLNQAAL VFDFAFTESY QPLPIENYLA VVGDRYPRLK EIEYQSYAEL FASTVAEVN KLEMDLHLKG WNVKPIEEIN RIAIESLHHR TTKEVVYCVW DWFEFITQGE EFDLSKQIAI LQQLFRNSVY G GPTVYALL ...String: MLTKQAVEPV RINARTTDVF DIFNVKQYVG ANPYLNQAAL VFDFAFTESY QPLPIENYLA VVGDRYPRLK EIEYQSYAEL FASTVAEVN KLEMDLHLKG WNVKPIEEIN RIAIESLHHR TTKEVVYCVW DWFEFITQGE EFDLSKQIAI LQQLFRNSVY G GPTVYALL RTANEKHIPA FYLWDEGLMQ YGYGKQQVRG IATTFDVDSH IDSDFTTQKD DCKKFLQELG FPVPQGDVVF SL AEAKEVA AEIGYPVAVK PVAGHKGIGV TADVQDEIEL EAAYDRAVAG IPLEEKICII VENSIAGHDY RLLCVNGRFV AAT ERKPAY VVGDGYSTIA ELIEKENFSP NRSDTPTSPM GKIRTDEAMH LYLEEQGLDL DSVIDRDRTI YLRKVANLSS GGFS IDATN RVHPDNIILA QDIAQHFRLT CLGIDIITND IGRSWKETSF GIIEINAAPG VYMHLKPAIG EPVDVTARIL ETFFE TEKN ARIPIITFNR VSIRQLQKLS DRILMSHPDW TIGAVCREGI LINRSEKILN RHYNTNVLNL LRNPKLDLLI AEYDED ALE AEGMFYHGSN LVVLEDPSEI EMILTRDVFS DSTVIIKQGR EITIKRKGLL EQYELEAEEL IEQVYLKEIG TISENLY FQ UniProtKB: RimK domain-containing protein ATP-grasp |
-Macromolecule #2: 4x(beta-Asp-Arg)
| Macromolecule | Name: 4x(beta-Asp-Arg) / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 1.103106 KDa |
| Sequence | String: (7ID)(7ID)(7ID)(7ID) |
-Macromolecule #3: 1-[(4-aminopyrimidin-5-yl)amino]-2,5-anhydro-1-deoxy-6-O-[(S)-hyd...
| Macromolecule | Name: 1-[(4-aminopyrimidin-5-yl)amino]-2,5-anhydro-1-deoxy-6-O-[(S)-hydroxy{[(R)-hydroxy(phosphonomethyl)phosphoryl]oxy}phosphoryl]-D-allitol type: ligand / ID: 3 / Number of copies: 1 / Formula: YHZ |
|---|---|
| Molecular weight | Theoretical: 494.225 Da |
| Chemical component information |  ChemComp-YHZ: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: PHOSPHOMETHYLPHOSPHONIC ACID ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOMETHYLPHOSPHONIC ACID ADENYLATE ESTER / type: ligand / ID: 5 / Number of copies: 1 / Formula: ACP |
|---|---|
| Molecular weight | Theoretical: 505.208 Da |
| Chemical component information |  ChemComp-ACP: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: -0.0025 µm / Nominal defocus min: -0.001 µm Bright-field microscopy / Nominal defocus max: -0.0025 µm / Nominal defocus min: -0.001 µm |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 80.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z
Z Y
Y X
X