[English] 日本語
 Yorodumi
Yorodumi- EMDB-29417: EM map of S. cerevisiae Rad24-RFC loading the 9-1-1 clamp onto a ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 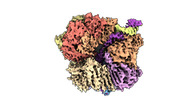 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
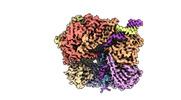
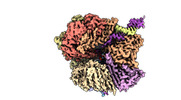
| Title | EM map of S. cerevisiae Rad24-RFC loading the 9-1-1 clamp onto a 5-nt gapped DNA (9-1-1 encircling fully bound DNA) | ||||||||||||
 Map data Map data | EM map of S. cerevisiae Rad24-RFC loading the 9-1-1 clamp onto a 5-nt gapped DNA (9-1-1 encircling fully bound DNA) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords |  DNA damage repair / Rad24-RFC / 9-1-1 clamp / DNA damage repair / Rad24-RFC / 9-1-1 clamp /  DNA clamp / alternative clamp loader / DNA damage signaling / DNA BINDING PROTEIN-DNA complex / CELL CYCLE-DNA complex DNA clamp / alternative clamp loader / DNA damage signaling / DNA BINDING PROTEIN-DNA complex / CELL CYCLE-DNA complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationmeiotic DNA integrity checkpoint signaling / checkpoint clamp complex / DNA clamp unloading / Rad17 RFC-like complex / Elg1 RFC-like complex / DNA replication factor C complex / Ctf18 RFC-like complex / DNA clamp loader activity / Polymerase switching / telomere maintenance via recombination ...meiotic DNA integrity checkpoint signaling / checkpoint clamp complex / DNA clamp unloading / Rad17 RFC-like complex / Elg1 RFC-like complex / DNA replication factor C complex / Ctf18 RFC-like complex / DNA clamp loader activity / Polymerase switching / telomere maintenance via recombination /  nuclease activity / DNA replication checkpoint signaling / Activation of ATR in response to replication stress / mitotic DNA replication checkpoint signaling / reciprocal meiotic recombination / nuclease activity / DNA replication checkpoint signaling / Activation of ATR in response to replication stress / mitotic DNA replication checkpoint signaling / reciprocal meiotic recombination /  recombinational repair / mitotic intra-S DNA damage checkpoint signaling / sister chromatid cohesion / mitotic sister chromatid cohesion / leading strand elongation / Gap-filling DNA repair synthesis and ligation in TC-NER / subtelomeric heterochromatin formation / recombinational repair / mitotic intra-S DNA damage checkpoint signaling / sister chromatid cohesion / mitotic sister chromatid cohesion / leading strand elongation / Gap-filling DNA repair synthesis and ligation in TC-NER / subtelomeric heterochromatin formation /  mismatch repair / mismatch repair /  telomere maintenance / meiotic cell cycle / DNA damage checkpoint signaling / nucleotide-excision repair / double-strand break repair via homologous recombination / DNA-templated DNA replication / double-strand break repair / site of double-strand break / telomere maintenance / meiotic cell cycle / DNA damage checkpoint signaling / nucleotide-excision repair / double-strand break repair via homologous recombination / DNA-templated DNA replication / double-strand break repair / site of double-strand break /  double-stranded DNA binding / damaged DNA binding / double-stranded DNA binding / damaged DNA binding /  chromosome, telomeric region / chromosome, telomeric region /  DNA repair / DNA repair /  chromatin binding / chromatin binding /  ATP hydrolysis activity / ATP hydrolysis activity /  DNA binding / DNA binding /  ATP binding / ATP binding /  nucleus / nucleus /  cytosol cytosolSimilarity search - Function | ||||||||||||
| Biological species | synthetic construct (others) /   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | ||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.04 Å cryo EM / Resolution: 3.04 Å | ||||||||||||
 Authors Authors | Zheng F / Georgescu R / Yao YN / O'Donnell ME / Li H | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2023 Journal: bioRxiv / Year: 2023Title: Structures of 9-1-1 DNA checkpoint clamp loading at gaps from start to finish and ramification to biology. Authors: Fengwei Zheng / Roxana E Georgescu / Nina Y Yao / Michael E O'Donnell / Huilin Li /  Abstract: Recent structural studies show the Rad24-RFC loads the 9-1-1 checkpoint clamp onto a recessed 5' end by binding the 5' DNA on Rad24 at an external surface site and threading the 3' ssDNA into the ...Recent structural studies show the Rad24-RFC loads the 9-1-1 checkpoint clamp onto a recessed 5' end by binding the 5' DNA on Rad24 at an external surface site and threading the 3' ssDNA into the well-established internal chamber and into 9-1-1. We find here that Rad24-RFC loads 9-1-1 onto DNA gaps in preference to a recessed 5' DNA end, thus presumably leaving 9-1-1 on a 3' ss/ds DNA after Rad24-RFC ejects from the 5' gap end and may explain reports of 9-1-1 directly functioning in DNA repair with various TLS polymerases, in addition to signaling the ATR kinase. To gain a deeper understanding of 9-1-1 loading at gaps we report high-resolution structures of Rad24-RFC during loading of 9-1-1 onto 10-nt and 5-nt gapped DNAs. At a 10-nt gap we captured five Rad24-RFC-9-1-1 loading intermediates in which the 9-1-1 DNA entry gate varies from fully open to fully closed around DNA using ATPγS, supporting the emerging view that ATP hydrolysis is not needed for clamp opening/closing, but instead for dissociation of the loader from the clamp encircling DNA. The structure of Rad24-RFC-9-1-1 at a 5-nt gap shows a 180° axially rotated 3'-dsDNA which orients the template strand to bridge the 3'- and 5'- junctions with a minimum 5-nt ssDNA. The structures reveal a unique loop on Rad24 that limits the length of dsDNA in the inner chamber, and inability to melt DNA ends unlike RFC, thereby explaining Rad24-RFC's preference for a preexisting ssDNA gap and suggesting a direct role in gap repair in addition to its checkpoint role. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29417.map.gz emd_29417.map.gz | 267.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29417-v30.xml emd-29417-v30.xml emd-29417.xml emd-29417.xml | 28.4 KB 28.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29417_fsc.xml emd_29417_fsc.xml | 13.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_29417.png emd_29417.png | 72.1 KB | ||
| Others |  emd_29417_half_map_1.map.gz emd_29417_half_map_1.map.gz emd_29417_half_map_2.map.gz emd_29417_half_map_2.map.gz | 262.2 MB 262.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29417 http://ftp.pdbj.org/pub/emdb/structures/EMD-29417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29417 | HTTPS FTP |
-Related structure data
| Related structure data |  8fs8MC  8fs3C  8fs4C  8fs5C  8fs6C  8fs7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29417.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29417.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM map of S. cerevisiae Rad24-RFC loading the 9-1-1 clamp onto a 5-nt gapped DNA (9-1-1 encircling fully bound DNA) | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map A
| File | emd_29417_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map B
| File | emd_29417_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Rad24-RFC-911 clamp-DNA
+Supramolecule #1: Rad24-RFC-911 clamp-DNA
+Supramolecule #2: DNA
+Supramolecule #3: Proteins complex
+Macromolecule #1: Checkpoint protein RAD24
+Macromolecule #2: Replication factor C subunit 4
+Macromolecule #3: Replication factor C subunit 3
+Macromolecule #4: Replication factor C subunit 2
+Macromolecule #5: Replication factor C subunit 5
+Macromolecule #6: DNA damage checkpoint control protein MEC3
+Macromolecule #7: DNA damage checkpoint control protein RAD17
+Macromolecule #8: DDC1 isoform 1
+Macromolecule #9: Template strand
+Macromolecule #10: Primer strand 1
+Macromolecule #11: Primer strand 2
+Macromolecule #12: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
+Macromolecule #13: MAGNESIUM ION
+Macromolecule #14: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 1.3 µm Bright-field microscopy / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 1.3 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 64.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



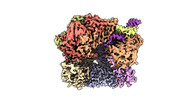
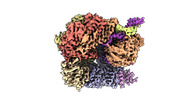
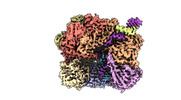






 Z
Z Y
Y X
X





















