[English] 日本語
 Yorodumi
Yorodumi- EMDB-29343: Cryo-EM structure of human TRPV6 in complex with the natural phyt... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human TRPV6 in complex with the natural phytoestrogen genistein | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationparathyroid hormone secretion / regulation of calcium ion-dependent exocytosis /  TRP channels / calcium ion import across plasma membrane / calcium ion homeostasis / TRP channels / calcium ion import across plasma membrane / calcium ion homeostasis /  calcium channel complex / calcium ion transmembrane transport / calcium channel complex / calcium ion transmembrane transport /  calcium channel activity / response to calcium ion / calcium ion transport ...parathyroid hormone secretion / regulation of calcium ion-dependent exocytosis / calcium channel activity / response to calcium ion / calcium ion transport ...parathyroid hormone secretion / regulation of calcium ion-dependent exocytosis /  TRP channels / calcium ion import across plasma membrane / calcium ion homeostasis / TRP channels / calcium ion import across plasma membrane / calcium ion homeostasis /  calcium channel complex / calcium ion transmembrane transport / calcium channel complex / calcium ion transmembrane transport /  calcium channel activity / response to calcium ion / calcium ion transport / calcium channel activity / response to calcium ion / calcium ion transport /  calmodulin binding / identical protein binding / calmodulin binding / identical protein binding /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.66 Å cryo EM / Resolution: 2.66 Å | ||||||||||||||||||
 Authors Authors | Neuberger A / Yelshanskaya MV / Nadezhdin KD / Sobolevsky AI | ||||||||||||||||||
| Funding support |  United States, United States,  Germany, 5 items Germany, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural mechanism of human oncochannel TRPV6 inhibition by the natural phytoestrogen genistein. Authors: Arthur Neuberger / Yury A Trofimov / Maria V Yelshanskaya / Kirill D Nadezhdin / Nikolay A Krylov / Roman G Efremov / Alexander I Sobolevsky /   Abstract: Calcium-selective oncochannel TRPV6 is the major driver of cell proliferation in human cancers. While significant effort has been invested in the development of synthetic TRPV6 inhibitors, natural ...Calcium-selective oncochannel TRPV6 is the major driver of cell proliferation in human cancers. While significant effort has been invested in the development of synthetic TRPV6 inhibitors, natural channel blockers have been largely neglected. Here we report the structure of human TRPV6 in complex with the plant-derived phytoestrogen genistein, extracted from Styphnolobium japonicum, that was shown to inhibit cell invasion and metastasis in cancer clinical trials. Despite the pharmacological value, the molecular mechanism of TRPV6 inhibition by genistein has remained enigmatic. We use cryo-EM combined with electrophysiology, calcium imaging, mutagenesis, and molecular dynamics simulations to show that genistein binds in the intracellular half of the TRPV6 pore and acts as an ion channel blocker and gating modifier. Genistein binding to the open channel causes pore closure and a two-fold symmetrical conformational rearrangement in the S4-S5 and S6-TRP helix regions. The unprecedented mechanism of TRPV6 inhibition by genistein uncovers new possibilities in structure-based drug design. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29343.map.gz emd_29343.map.gz | 59.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29343-v30.xml emd-29343-v30.xml emd-29343.xml emd-29343.xml | 20.7 KB 20.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29343.png emd_29343.png | 84.8 KB | ||
| Others |  emd_29343_half_map_1.map.gz emd_29343_half_map_1.map.gz emd_29343_half_map_2.map.gz emd_29343_half_map_2.map.gz | 59 MB 59.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29343 http://ftp.pdbj.org/pub/emdb/structures/EMD-29343 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29343 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29343 | HTTPS FTP |
-Related structure data
| Related structure data |  8foaMC  8fobC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29343.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29343.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_29343_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
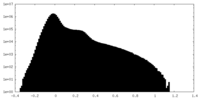
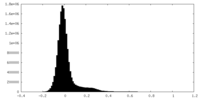
| Density Histograms |
-Half map: #2
| File | emd_29343_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : sample 1
| Entire | Name: sample 1 |
|---|---|
| Components |
|
-Supramolecule #1: sample 1
| Supramolecule | Name: sample 1 / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 83.22 KDa |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 6
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 6 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 83.302828 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGLSLPKEKG LILCLWSKFC RWFQRRESWA QSRDEQNLLQ QKRIWESPLL LAAKDNDVQA LNKLLKYEDC KVHQRGAMGE TALHIAALY DNLEAAMVLM EAAPELVFEP MTSELYEGQT ALHIAVVNQN MNLVRALLAR RASVSARATG TAFRRSPCNL I YFGEHPLS ...String: MGLSLPKEKG LILCLWSKFC RWFQRRESWA QSRDEQNLLQ QKRIWESPLL LAAKDNDVQA LNKLLKYEDC KVHQRGAMGE TALHIAALY DNLEAAMVLM EAAPELVFEP MTSELYEGQT ALHIAVVNQN MNLVRALLAR RASVSARATG TAFRRSPCNL I YFGEHPLS FAACVNSEEI VRLLIEHGAD IRAQDSLGNT VLHILILQPN KTFACQMYNL LLSYDRHGDH LQPLDLVPNH QG LTPFKLA GVEGNTVMFQ HLMQKRKHTQ WTYGPLTSTL YDLTEIDSSG DEQSLLELII TTKKREARQI LDQTPVKELV SLK WKRYGR PYFCMLGAIY LLYIICFTMC CIYRPLKPRT NNRTSPRDNT LLQQKLLQEA YMTPKDDIRL VGELVTVIGA IIIL LVEVP DIFRMGVTRF FGQTILGGPF HVLIITYAFM VLVTMVMRLI SASGEVVPMS FALVLGWCNV MYFARGFQML GPFTI MIQK MIFGDLMRFC WLMAVVILGF ASAFYIIFQT EDPEELGHFY DYPMALFSTF ELFLTIIDGP ANYNVDLPFM YSITYA AFA IIATLLMLNL LIAMMGDTHW RVAHERDELW RAQIVATTVM LERKLPRCLW PRSGICGREY GLGDRWFLRV EDRQDLN RQ RIQRYAQAFH TRGSEDLDKD SVEKLELGCP FSPHLSLPMP SVSRSTSRSS ANWERLRQGT LRRDLRGIIN RGLEDGES W EYQI |
-Macromolecule #2: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 2 / Number of copies: 12 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Macromolecule #3: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 3 / Number of copies: 36 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Macromolecule #4: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #5: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #6: GENISTEIN
| Macromolecule | Name: GENISTEIN / type: ligand / ID: 6 / Number of copies: 2 / Formula: GEN |
|---|---|
| Molecular weight | Theoretical: 270.237 Da |
| Chemical component information |  ChemComp-GEN: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.36 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||||||||
| Details | human TRPV6 |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 3630 / Average exposure time: 2.5 sec. / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 2343818 |
|---|---|
| Initial angle assignment | Type: OTHER / Software - Name: cryoSPARC (ver. 3.3) |
| Final 3D classification | Software - Name: cryoSPARC (ver. 3.3) |
| Final angle assignment | Type: OTHER / Software - Name: cryoSPARC (ver. 3.3) |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.66 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 3.3) / Number images used: 196340 |
 Movie
Movie Controller
Controller





 Z
Z Y
Y X
X