+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human pannexin 2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationElectric Transmission Across Gap Junctions / wide pore channel activity / positive regulation of interleukin-1 production /  gap junction / monoatomic cation transport / monoatomic ion transmembrane transport / response to ischemia / cell-cell signaling / gap junction / monoatomic cation transport / monoatomic ion transmembrane transport / response to ischemia / cell-cell signaling /  electron transfer activity / electron transfer activity /  periplasmic space ...Electric Transmission Across Gap Junctions / wide pore channel activity / positive regulation of interleukin-1 production / periplasmic space ...Electric Transmission Across Gap Junctions / wide pore channel activity / positive regulation of interleukin-1 production /  gap junction / monoatomic cation transport / monoatomic ion transmembrane transport / response to ischemia / cell-cell signaling / gap junction / monoatomic cation transport / monoatomic ion transmembrane transport / response to ischemia / cell-cell signaling /  electron transfer activity / electron transfer activity /  periplasmic space / iron ion binding / periplasmic space / iron ion binding /  heme binding / structural molecule activity / heme binding / structural molecule activity /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.92 Å cryo EM / Resolution: 3.92 Å | |||||||||
 Authors Authors | He Z / Yuan P | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural and functional analysis of human pannexin 2 channel. Authors: Zhihui He / Yonghui Zhao / Michael J Rau / James A J Fitzpatrick / Rajan Sah / Hongzhen Hu / Peng Yuan /  Abstract: The pannexin 2 channel (PANX2) participates in multiple physiological processes including skin homeostasis, neuronal development, and ischemia-induced brain injury. However, the molecular basis of ...The pannexin 2 channel (PANX2) participates in multiple physiological processes including skin homeostasis, neuronal development, and ischemia-induced brain injury. However, the molecular basis of PANX2 channel function remains largely unknown. Here, we present a cryo-electron microscopy structure of human PANX2, which reveals pore properties contrasting with those of the intensely studied paralog PANX1. The extracellular selectivity filter, defined by a ring of basic residues, more closely resembles that of the distantly related volume-regulated anion channel (VRAC) LRRC8A, rather than PANX1. Furthermore, we show that PANX2 displays a similar anion permeability sequence as VRAC, and that PANX2 channel activity is inhibited by a commonly used VRAC inhibitor, DCPIB. Thus, the shared channel properties between PANX2 and VRAC may complicate dissection of their cellular functions through pharmacological manipulation. Collectively, our structural and functional analysis provides a framework for development of PANX2-specific reagents that are needed for better understanding of channel physiology and pathophysiology. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28902.map.gz emd_28902.map.gz | 78.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28902-v30.xml emd-28902-v30.xml emd-28902.xml emd-28902.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28902.png emd_28902.png | 156.5 KB | ||
| Others |  emd_28902_half_map_1.map.gz emd_28902_half_map_1.map.gz emd_28902_half_map_2.map.gz emd_28902_half_map_2.map.gz | 77.5 MB 77.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28902 http://ftp.pdbj.org/pub/emdb/structures/EMD-28902 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28902 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28902 | HTTPS FTP |
-Related structure data
| Related structure data |  8f7cMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28902.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28902.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.9 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
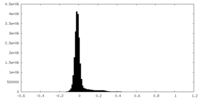
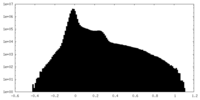
-Half map: #2
| File | emd_28902_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
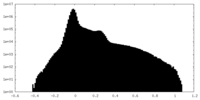
| Projections & Slices |
| ||||||||||||
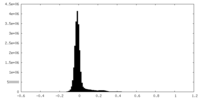
| Density Histograms |
-Half map: #1
| File | emd_28902_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human pannexin 2
| Entire | Name: human pannexin 2 |
|---|---|
| Components |
|
-Supramolecule #1: human pannexin 2
| Supramolecule | Name: human pannexin 2 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 380 KDa |
-Macromolecule #1: Pannexin-2, Soluble cytochrome b562 fusion
| Macromolecule | Name: Pannexin-2, Soluble cytochrome b562 fusion / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 55.033406 KDa |
| Recombinant expression | Organism:   Komagataella pastoris (fungus) Komagataella pastoris (fungus) |
| Sequence | String: MHHLLEQSAD MATALLAGEK LRELILPGAQ DDKAGALAAL LLQLKLELPF DRVVTIGTVL VPILLVTLVF TKNFAEEPIY CYTPHNFTR DQALYARGYC WTELRDALPG VDASLWPSLF EHKFLPYALL AFAAIMYVPA LGWEFLASTR LTSELNFLLQ E IDNCYHRA ...String: MHHLLEQSAD MATALLAGEK LRELILPGAQ DDKAGALAAL LLQLKLELPF DRVVTIGTVL VPILLVTLVF TKNFAEEPIY CYTPHNFTR DQALYARGYC WTELRDALPG VDASLWPSLF EHKFLPYALL AFAAIMYVPA LGWEFLASTR LTSELNFLLQ E IDNCYHRA AEGRAPKIEK QIQSKGPGIT EREKREIIEN AEKEKSPEQN LFEKYLERRG RSNFLAKLYL ARHVLILLLS AV PISYLCT YYATQKQNEF TCALGASPDG AAGAGPAVRV SCKLPSVQLQ RIIAGVDIVL LCVMNLIILV NLIHLFIFRK SNF IFDKLH KVGIKTRRQW RRSQFCDINI LAMFCNENRD HIKSLNRLDF ITNESDADLE DNWETLNDNL KVIEKADNAA QVKD ALTKM RAAALDAQKA TPPKLEDKSP DSPEMKDFRH GFDILVGQID DALKLANEGK VKEAQAAAEQ LKTTRNAYIQ KYLSN SLEV LFQ |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7.5 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 20mM Tris-HCL, 150mM NaCl, 40uM GDN, 1mM Fluorinated Fos-Choline-8 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 49.0 e/Å2 |
- Image processing
Image processing
| Particle selection | Number selected: 467552 |
|---|---|
| Initial angle assignment | Type: NOT APPLICABLE |
| Final 3D classification | Number classes: 4 / Avg.num./class: 16885 / Software - Name: RELION (ver. 3.0) |
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Number classes used: 1 / Applied symmetry - Point group: C7 (7 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 3.92 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 25191 ) / Resolution.type: BY AUTHOR / Resolution: 3.92 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 25191 |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 78.73 |
|---|---|
| Output model |  PDB-8f7c: |
 Movie
Movie Controller
Controller










 Z
Z Y
Y X
X