[English] 日本語
 Yorodumi
Yorodumi- EMDB-28830: ELIC with Propylamine in saposin nanodiscs with 2:1:1 POPC:POPE:POPG -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ELIC with Propylamine in saposin nanodiscs with 2:1:1 POPC:POPE:POPG | |||||||||
 Map data Map data | Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ELIC /  ion channel / pLGIC / ion channel / pLGIC /  Structural Protein / Structural Protein /  Membrane Protein / Membrane Protein /  TRANSPORT PROTEIN TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationextracellular ligand-gated monoatomic ion channel activity / transmembrane signaling receptor activity /  membrane / identical protein binding membrane / identical protein bindingSimilarity search - Function | |||||||||
| Biological species |   Dickeya dadantii (bacteria) Dickeya dadantii (bacteria) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.28 Å cryo EM / Resolution: 3.28 Å | |||||||||
 Authors Authors | Dalal V / Arcario MJ / Petroff II JT / Deitzen NM / Tan BK / Brannigan G / Cheng WWL | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Lipid nanodisc scaffold and size alter the structure of a pentameric ligand-gated ion channel. Authors: Vikram Dalal / Mark J Arcario / John T Petroff / Brandon K Tan / Noah M Dietzen / Michael J Rau / James A J Fitzpatrick / Grace Brannigan / Wayland W L Cheng /  Abstract: Lipid nanodiscs have become a standard tool for studying membrane proteins, including using single particle cryo-electron microscopy (cryo-EM). We find that reconstituting the pentameric ligand-gated ...Lipid nanodiscs have become a standard tool for studying membrane proteins, including using single particle cryo-electron microscopy (cryo-EM). We find that reconstituting the pentameric ligand-gated ion channel (pLGIC), Erwinia ligand-gated ion channel (ELIC), in different nanodiscs produces distinct structures by cryo-EM. The effect of the nanodisc on ELIC structure extends to the extracellular domain and agonist binding site. Additionally, molecular dynamic simulations indicate that nanodiscs of different size impact ELIC structure and that the nanodisc scaffold directly interacts with ELIC. These findings suggest that the nanodisc plays a crucial role in determining the structure of pLGICs, and that reconstitution of ion channels in larger nanodiscs may better approximate a lipid membrane environment. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28830.map.gz emd_28830.map.gz | 78.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28830-v30.xml emd-28830-v30.xml emd-28830.xml emd-28830.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28830_fsc.xml emd_28830_fsc.xml | 10 KB | Display |  FSC data file FSC data file |
| Images |  emd_28830.png emd_28830.png | 34.9 KB | ||
| Filedesc metadata |  emd-28830.cif.gz emd-28830.cif.gz | 6 KB | ||
| Others |  emd_28830_additional_1.map.gz emd_28830_additional_1.map.gz emd_28830_half_map_1.map.gz emd_28830_half_map_1.map.gz emd_28830_half_map_2.map.gz emd_28830_half_map_2.map.gz | 64.4 MB 65.1 MB 65.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28830 http://ftp.pdbj.org/pub/emdb/structures/EMD-28830 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28830 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28830 | HTTPS FTP |
-Related structure data
| Related structure data |  8f33MC  8f32C  8f34C  8f35C  8twvC  8twzC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28830.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28830.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.657 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin
| File | emd_28830_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin | ||||||||||||
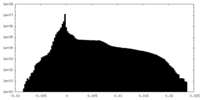
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin
| File | emd_28830_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin | ||||||||||||
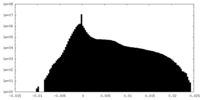
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin
| File | emd_28830_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Propylamine bound Wild Type ELIC in 2:1:1 POPC: POPE: POPG in saposin | ||||||||||||
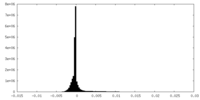
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ELIC with Propylamine in saposin nanodiscs with 2:1:1 POPC:POPE:POPG
| Entire | Name: ELIC with Propylamine in saposin nanodiscs with 2:1:1 POPC:POPE:POPG |
|---|---|
| Components |
|
-Supramolecule #1: ELIC with Propylamine in saposin nanodiscs with 2:1:1 POPC:POPE:POPG
| Supramolecule | Name: ELIC with Propylamine in saposin nanodiscs with 2:1:1 POPC:POPE:POPG type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Dickeya dadantii (bacteria) Dickeya dadantii (bacteria) |
-Macromolecule #1: Erwinia chrysanthemi ligand-gated ion channel
| Macromolecule | Name: Erwinia chrysanthemi ligand-gated ion channel / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Dickeya dadantii (bacteria) Dickeya dadantii (bacteria) |
| Molecular weight | Theoretical: 36.879 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: APADNAADAR PVDVSVSIFI NKIYGVNTLE QTYKVDGYIV AQWTGKPRKT PGDKPLIVEN TQIERWINNG LWVPALEFIN VVGSPDTGN KRLMLFPDGR VIYNARFLGS FSNDMDFRLF PFDRQQFVLE LEPFSYNNQQ LRFSDIQVYT ENIDNEEIDE W WIRGKAST ...String: APADNAADAR PVDVSVSIFI NKIYGVNTLE QTYKVDGYIV AQWTGKPRKT PGDKPLIVEN TQIERWINNG LWVPALEFIN VVGSPDTGN KRLMLFPDGR VIYNARFLGS FSNDMDFRLF PFDRQQFVLE LEPFSYNNQQ LRFSDIQVYT ENIDNEEIDE W WIRGKAST HISDIRYDHL SSVQPNQNEF SRITVRIDAV RNPSYYLWSF ILPLGLIIAA SWSVFWLESF SERLQTSFTL ML TVVAYAF YTSNILPRLP YTTVIDQMII AGYGSIFAAI LLIIFAHHRQ ANGVEDDLLI QRCRLAFPLG FLAIGCVLVI RGI TL UniProtKB: Gamma-aminobutyric-acid receptor subunit beta-1 |
-Macromolecule #2: 3-AMINOPROPANE
| Macromolecule | Name: 3-AMINOPROPANE / type: ligand / ID: 2 / Number of copies: 5 / Formula: 3CN |
|---|---|
| Molecular weight | Theoretical: 59.11 Da |
| Chemical component information | 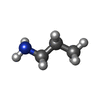 ChemComp-3CN: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: OTHER / Pretreatment - Pressure: 9.332 kPa / Details: O2 27.5 sccm H2 6.4 sccm |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Blot for 2 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 96000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 0.01 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 96000 Bright-field microscopy / Cs: 0.01 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 96000 |
| Specialist optics | Spherical aberration corrector: Microscope was equipped with a Cs corrector |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Average exposure time: 4.28 sec. / Average electron dose: 47.55 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8f33: |
 Movie
Movie Controller
Controller










 Z
Z Y
Y X
X