+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | RMC-5552 in complex with mTORC1 and FKBP12 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationRNA polymerase III type 2 promoter sequence-specific DNA binding / positive regulation of cytoplasmic translational initiation / RNA polymerase III type 1 promoter sequence-specific DNA binding / positive regulation of pentose-phosphate shunt / T-helper 1 cell lineage commitment / regulation of locomotor rhythm / positive regulation of wound healing, spreading of epidermal cells / cellular response to leucine starvation / TFIIIC-class transcription factor complex binding /  TORC2 complex ...RNA polymerase III type 2 promoter sequence-specific DNA binding / positive regulation of cytoplasmic translational initiation / RNA polymerase III type 1 promoter sequence-specific DNA binding / positive regulation of pentose-phosphate shunt / T-helper 1 cell lineage commitment / regulation of locomotor rhythm / positive regulation of wound healing, spreading of epidermal cells / cellular response to leucine starvation / TFIIIC-class transcription factor complex binding / TORC2 complex ...RNA polymerase III type 2 promoter sequence-specific DNA binding / positive regulation of cytoplasmic translational initiation / RNA polymerase III type 1 promoter sequence-specific DNA binding / positive regulation of pentose-phosphate shunt / T-helper 1 cell lineage commitment / regulation of locomotor rhythm / positive regulation of wound healing, spreading of epidermal cells / cellular response to leucine starvation / TFIIIC-class transcription factor complex binding /  TORC2 complex / TORC2 complex /  regulation of membrane permeability / heart valve morphogenesis / negative regulation of lysosome organization / regulation of membrane permeability / heart valve morphogenesis / negative regulation of lysosome organization /  macrolide binding / RNA polymerase III type 3 promoter sequence-specific DNA binding / macrolide binding / RNA polymerase III type 3 promoter sequence-specific DNA binding /  TORC1 complex / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / calcineurin-NFAT signaling cascade / TORC1 complex / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / calcineurin-NFAT signaling cascade /  activin receptor binding / cytoplasmic side of membrane / activin receptor binding / cytoplasmic side of membrane /  regulation of autophagosome assembly / TORC1 signaling / positive regulation of odontoblast differentiation / voluntary musculoskeletal movement / regulation of osteoclast differentiation / positive regulation of keratinocyte migration / regulation of autophagosome assembly / TORC1 signaling / positive regulation of odontoblast differentiation / voluntary musculoskeletal movement / regulation of osteoclast differentiation / positive regulation of keratinocyte migration /  transforming growth factor beta receptor binding / TGFBR1 LBD Mutants in Cancer / cellular response to L-leucine / signaling receptor inhibitor activity / MTOR signalling / Amino acids regulate mTORC1 / cellular response to nutrient / type I transforming growth factor beta receptor binding / energy reserve metabolic process / Energy dependent regulation of mTOR by LKB1-AMPK / nucleus localization / negative regulation of activin receptor signaling pathway / ruffle organization / protein serine/threonine kinase inhibitor activity / heart trabecula formation / negative regulation of cell size / cellular response to osmotic stress / positive regulation of osteoclast differentiation / transforming growth factor beta receptor binding / TGFBR1 LBD Mutants in Cancer / cellular response to L-leucine / signaling receptor inhibitor activity / MTOR signalling / Amino acids regulate mTORC1 / cellular response to nutrient / type I transforming growth factor beta receptor binding / energy reserve metabolic process / Energy dependent regulation of mTOR by LKB1-AMPK / nucleus localization / negative regulation of activin receptor signaling pathway / ruffle organization / protein serine/threonine kinase inhibitor activity / heart trabecula formation / negative regulation of cell size / cellular response to osmotic stress / positive regulation of osteoclast differentiation /  terminal cisterna / terminal cisterna /  ryanodine receptor complex / ryanodine receptor complex /  I-SMAD binding / enzyme-substrate adaptor activity / regulation of amyloid precursor protein catabolic process / I-SMAD binding / enzyme-substrate adaptor activity / regulation of amyloid precursor protein catabolic process /  anoikis / cardiac muscle cell development / negative regulation of protein localization to nucleus / positive regulation of transcription by RNA polymerase III / protein maturation by protein folding / anoikis / cardiac muscle cell development / negative regulation of protein localization to nucleus / positive regulation of transcription by RNA polymerase III / protein maturation by protein folding /  regulation of myelination / negative regulation of calcineurin-NFAT signaling cascade / 'de novo' protein folding / ventricular cardiac muscle tissue morphogenesis / regulation of myelination / negative regulation of calcineurin-NFAT signaling cascade / 'de novo' protein folding / ventricular cardiac muscle tissue morphogenesis /  Macroautophagy / Macroautophagy /  regulation of cell size / negative regulation of macroautophagy / lysosome organization / positive regulation of oligodendrocyte differentiation / negative regulation of phosphoprotein phosphatase activity / regulation of cell size / negative regulation of macroautophagy / lysosome organization / positive regulation of oligodendrocyte differentiation / negative regulation of phosphoprotein phosphatase activity /  FK506 binding / positive regulation of actin filament polymerization / protein kinase activator activity / positive regulation of myotube differentiation / behavioral response to pain / oligodendrocyte differentiation / FK506 binding / positive regulation of actin filament polymerization / protein kinase activator activity / positive regulation of myotube differentiation / behavioral response to pain / oligodendrocyte differentiation /  TOR signaling / mTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / Constitutive Signaling by AKT1 E17K in Cancer / germ cell development / TOR signaling / mTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / Constitutive Signaling by AKT1 E17K in Cancer / germ cell development /  social behavior / cellular response to nutrient levels / CD28 dependent PI3K/Akt signaling / positive regulation of translational initiation / positive regulation of phosphoprotein phosphatase activity / neuronal action potential / Calcineurin activates NFAT / HSF1-dependent transactivation / positive regulation of TOR signaling / positive regulation of G1/S transition of mitotic cell cycle / social behavior / cellular response to nutrient levels / CD28 dependent PI3K/Akt signaling / positive regulation of translational initiation / positive regulation of phosphoprotein phosphatase activity / neuronal action potential / Calcineurin activates NFAT / HSF1-dependent transactivation / positive regulation of TOR signaling / positive regulation of G1/S transition of mitotic cell cycle /  regulation of macroautophagy / positive regulation of epithelial to mesenchymal transition / regulation of macroautophagy / positive regulation of epithelial to mesenchymal transition /  endomembrane system / endomembrane system /  regulation of immune response / 'de novo' pyrimidine nucleobase biosynthetic process / response to amino acid / protein peptidyl-prolyl isomerization / supramolecular fiber organization / positive regulation of lamellipodium assembly / phagocytic vesicle / regulation of cellular response to heat / positive regulation of lipid biosynthetic process / heart morphogenesis / regulation of ryanodine-sensitive calcium-release channel activity / cardiac muscle contraction regulation of immune response / 'de novo' pyrimidine nucleobase biosynthetic process / response to amino acid / protein peptidyl-prolyl isomerization / supramolecular fiber organization / positive regulation of lamellipodium assembly / phagocytic vesicle / regulation of cellular response to heat / positive regulation of lipid biosynthetic process / heart morphogenesis / regulation of ryanodine-sensitive calcium-release channel activity / cardiac muscle contractionSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.86 Å cryo EM / Resolution: 2.86 Å | |||||||||
 Authors Authors | Tomlinson ACA / Yano JK | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: J Med Chem / Year: 2023 Journal: J Med Chem / Year: 2023Title: Discovery of RMC-5552, a Selective Bi-Steric Inhibitor of mTORC1, for the Treatment of mTORC1-Activated Tumors. Authors: G Leslie Burnett / Yu C Yang / James B Aggen / Jennifer Pitzen / Micah K Gliedt / Chris M Semko / Abby Marquez / James W Evans / Gang Wang / Walter S Won / Aidan C A Tomlinson / Gert Kiss / ...Authors: G Leslie Burnett / Yu C Yang / James B Aggen / Jennifer Pitzen / Micah K Gliedt / Chris M Semko / Abby Marquez / James W Evans / Gang Wang / Walter S Won / Aidan C A Tomlinson / Gert Kiss / Christos Tzitzilonis / Arun P Thottumkara / James Cregg / Kevin T Mellem / Jong S Choi / Julie C Lee / Yongyuan Zhao / Bianca J Lee / Justin G Meyerowitz / John E Knox / Jingjing Jiang / Zhican Wang / David Wildes / Zhengping Wang / Mallika Singh / Jacqueline A M Smith / Adrian L Gill /  Abstract: Hyperactivation of mTOR kinase by mutations in the PI3K/mTOR pathway or by crosstalk with other mutant cancer drivers, such as RAS, is a feature of many tumors. Multiple allosteric inhibitors of ...Hyperactivation of mTOR kinase by mutations in the PI3K/mTOR pathway or by crosstalk with other mutant cancer drivers, such as RAS, is a feature of many tumors. Multiple allosteric inhibitors of mTORC1 and orthosteric dual inhibitors of mTORC1 and mTORC2 have been developed as anticancer drugs, but their clinical utility has been limited. To address these limitations, we have developed a novel class of "bi-steric inhibitors" that interact with both the orthosteric and the allosteric binding sites in order to deepen the inhibition of mTORC1 while also preserving selectivity for mTORC1 over mTORC2. In this report, we describe the discovery and preclinical profile of the development candidate RMC-5552 and the in vivo preclinical tool compound RMC-6272. We also present evidence that selective inhibition of mTORC1 in combination with covalent inhibition of KRAS shows increased antitumor activity in a preclinical model of mutant NSCLC that exhibits resistance to KRAS inhibitor monotherapy. #1:  Journal: Acta Crystallogr D Biol Crystallogr / Year: 2012 Journal: Acta Crystallogr D Biol Crystallogr / Year: 2012Title: Towards automated crystallographic structure refinement with phenix.refine. Authors: Afonine PV / Grosse-Kunstleve RW / Echols N / Headd JJ / Moriarty NW / Mustyakimov M / Terwilliger TC / Urzhumtsev A / Zwart PH / Adams PD | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28551.map.gz emd_28551.map.gz | 165.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28551-v30.xml emd-28551-v30.xml emd-28551.xml emd-28551.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28551.png emd_28551.png | 88.9 KB | ||
| Others |  emd_28551_half_map_1.map.gz emd_28551_half_map_1.map.gz emd_28551_half_map_2.map.gz emd_28551_half_map_2.map.gz | 140.9 MB 140.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28551 http://ftp.pdbj.org/pub/emdb/structures/EMD-28551 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28551 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28551 | HTTPS FTP |
-Related structure data
| Related structure data |  8eraMC  8er6C  8er7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28551.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28551.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_28551_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
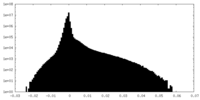
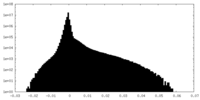
| Density Histograms |
-Half map: #1
| File | emd_28551_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
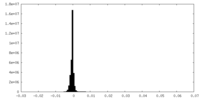
| Density Histograms |
- Sample components
Sample components
-Entire : RMC-5552-mTORC1-FKBP12
| Entire | Name: RMC-5552-mTORC1-FKBP12 |
|---|---|
| Components |
|
-Supramolecule #1: RMC-5552-mTORC1-FKBP12
| Supramolecule | Name: RMC-5552-mTORC1-FKBP12 / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Serine/threonine-protein kinase mTOR
| Macromolecule | Name: Serine/threonine-protein kinase mTOR / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number:  non-specific serine/threonine protein kinase non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 289.257969 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MLGTGPAAAT TAATTSSNVS VLQQFASGLK SRNEETRAKA AKELQHYVTM ELREMSQEES TRFYDQLNHH IFELVSSSDA NERKGGILA IASLIGVEGG NATRIGRFAN YLRNLLPSND PVVMEMASKA IGRLAMAGDT FTAEYVEFEV KRALEWLGAD R NEGRRHAA ...String: MLGTGPAAAT TAATTSSNVS VLQQFASGLK SRNEETRAKA AKELQHYVTM ELREMSQEES TRFYDQLNHH IFELVSSSDA NERKGGILA IASLIGVEGG NATRIGRFAN YLRNLLPSND PVVMEMASKA IGRLAMAGDT FTAEYVEFEV KRALEWLGAD R NEGRRHAA VLVLRELAIS VPTFFFQQVQ PFFDNIFVAV WDPKQAIREG AVAALRACLI LTTQREPKEM QKPQWYRHTF EE AEKGFDE TLAKEKGMNR DDRIHGALLI LNELVRISSM EGERLREEME EITQQQLVHD KYCKDLMGFG TKPRHITPFT SFQ AVQPQQ SNALVGLLGY SSHQGLMGFG TSPSPAKSTL VESRCCRDLM EEKFDQVCQW VLKCRNSKNS LIQMTILNLL PRLA AFRPS AFTDTQYLQD TMNHVLSCVK KEKERTAAFQ ALGLLSVAVR SEFKVYLPRV LDIIRAALPP KDFAHKRQKA MQVDA TVFT CISMLARAMG PGIQQDIKEL LEPMLAVGLS PALTAVLYDL SRQIPQLKKD IQDGLLKMLS LVLMHKPLRH PGMPKG LAH QLASPGLTTL PEASDVGSIT LALRTLGSFE FEGHSLTQFV RHCADHFLNS EHKEIRMEAA RTCSRLLTPS IHLISGH AH VVSQTAVQVV ADVLSKLLVV GITDPDPDIR YCVLASLDER FDAHLAQAEN LQALFVALND QVFEIRELAI CTVGRLSS M NPAFVMPFLR KMLIQILTEL EHSGIGRIKE QSARMLGHLV SNAPRLIRPY MEPILKALIL KLKDPDPDPN PGVINNVLA TIGELAQVSG LEMRKWVDEL FIIIMDMLQD SSLLAKRQVA LWTLGQLVAS TGYVVEPYRK YPTLLEVLLN FLKTEQNQGT RREAIRVLG LLGALDPYKH KVNIGMIDQS RDASAVSLSE SKSSQDSSDY STSEMLVNMG NLPLDEFYPA VSMVALMRIF R DQSLSHHH TMVVQAITFI FKSLGLKCVQ FLPQVMPTFL NVIRVCDGAI REFLFQQLGM LVSFVKSHIR PYMDEIVTLM RE FWVMNTS IQSTIILLIE QIVVALGGEF KLYLPQLIPH MLRVFMHDNS PGRIVSIKLL AAIQLFGANL DDYLHLLLPP IVK LFDAPE APLPSRKAAL ETVDRLTESL DFTDYASRII HPIVRTLDQS PELRSTAMDT LSSLVFQLGK KYQIFIPMVN KVLV RHRIN HQRYDVLICR IVKGYTLADE EEDPLIYQHR MLRSGQGDAL ASGPVETGPM KKLHVSTINL QKAWGAARRV SKDDW LEWL RRLSLELLKD SSSPSLRSCW ALAQAYNPMA RDLFNAAFVS CWSELNEDQQ DELIRSIELA LTSQDIAEVT QTLLNL AEF MEHSDKGPLP LRDDNGIVLL GERAAKCRAY AKALHYKELE FQKGPTPAIL ESLISINNKL QQPEAAAGVL EYAMKHF GE LEIQATWYEK LHEWEDALVA YDKKMDTNKD DPELMLGRMR CLEALGEWGQ LHQQCCEKWT LVNDETQAKM ARMAAAAA W GLGQWDSMEE YTCMIPRDTH DGAFYRAVLA LHQDLFSLAQ QCIDKARDLL DAELTAMAGE SYSRAYGAMV SCHMLSELE EVIQYKLVPE RREIIRQIWW ERLQGCQRIV EDWQKILMVR SLVVSPHEDM RTWLKYASLC GKSGRLALAH KTLVLLLGVD PSRQLDHPL PTVHPQVTYA YMKNMWKSAR KIDAFQHMQH FVQTMQQQAQ HAIATEDQQH KQELHKLMAR CFLKLGEWQL N LQGINEST IPKVLQYYSA ATEHDRSWYK AWHAWAVMNF EAVLHYKHQN QARDEKKKLR HASGANITNA TTAATTAATA TT TASTEGS NSESEAESTE NSPTPSPLQK KVTEDLSKTL LMYTVPAVQG FFRSISLSRG NNLQDTLRVL TLWFDYGHWP DVN EALVEG VKAIQIDTWL QVIPQLIARI DTPRPLVGRL IHQLLTDIGR YHPQALIYPL TVASKSTTTA RHNAANKILK NMCE HSNTL VQQAMMVSEE LIRVAILWHE MWHEGLEEAS RLYFGERNVK GMFEVLEPLH AMMERGPQTL KETSFNQAYG RDLME AQEW CRKYMKSGNV KDLTQAWDLY YHVFRRISKQ LPQLTSLELQ YVSPKLLMCR DLELAVPGTY DPNQPIIRIQ SIAPSL QVI TSKQRPRKLT LMGSNGHEFV FLLKGHEDLR QDERVMQLFG LVNTLLANDP TSLRKNLSIQ RYAVIPLSTN SGLIGWV PH CDTLHALIRD YREKKKILLN IEHRIMLRMA PDYDHLTLMQ KVEVFEHAVN NTAGDDLAKL LWLKSPSSEV WFDRRTNY T RSLAVMSMVG YILGLGDRHP SNLMLDRLSG KILHIDFGDC FEVAMTREKF PEKIPFRLTR MLTNAMEVTG LDGNYRITC HTVMEVLREH KDSVMAVLEA FVYDPLLNWR LMDTNTKGNK RSRTRTDSYS AGQSVEILDG VELGEPAHKK TGTTVPESIH SFIGDGLVK PEALNKKAIQ IINRVRDKLT GRDFSHDDTL DVPTQVELLI KQATSHENLC QCYIGWCPFW |
-Macromolecule #2: Peptidyl-prolyl cis-trans isomerase FKBP1A
| Macromolecule | Name: Peptidyl-prolyl cis-trans isomerase FKBP1A / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number:  peptidylprolyl isomerase peptidylprolyl isomerase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.923586 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: SGVQVETISP GDGRTFPKRG QTCVVHYTGM LEDGKKFDSS RDRNKPFKFM LGKQEVIRGW EEGVAQMSVG QRAKLTISPD YAYGATGHP GIIPPHATLV FDVELLKLE |
-Macromolecule #3: Target of rapamycin complex subunit LST8
| Macromolecule | Name: Target of rapamycin complex subunit LST8 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 35.91009 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MNTSPGTVGS DPVILATAGY DHTVRFWQAH SGICTRTVQH QDSQVNALEV TPDRSMIAAA GYQHIRMYDL NSNNPNPIIS YDGVNKNIA SVGFHEDGRW MYTGGEDCTA RIWDLRSRNL QCQRIFQVNA PINCVCLHPN QAELIVGDQS GAIHIWDLKT D HNEQLIPE ...String: MNTSPGTVGS DPVILATAGY DHTVRFWQAH SGICTRTVQH QDSQVNALEV TPDRSMIAAA GYQHIRMYDL NSNNPNPIIS YDGVNKNIA SVGFHEDGRW MYTGGEDCTA RIWDLRSRNL QCQRIFQVNA PINCVCLHPN QAELIVGDQS GAIHIWDLKT D HNEQLIPE PEVSITSAHI DPDASYMAAV NSTGNCYVWN LTGGIGDEVT QLIPKTKIPA HTRYALQCRF SPDSTLLATC SA DQTCKIW RTSNFSLMTE LSIKSGNPGE SSRGWMWGCA FSGDSQYIVT ASSDNLARLW CVETGEIKRE YGGHQKAVVC LAF NDSVLG |
-Macromolecule #4: Regulatory-associated protein of mTOR
| Macromolecule | Name: Regulatory-associated protein of mTOR / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 149.200016 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MESEMLQSPL LGLGEEDEAD LTDWNLPLAF MKKRHCEKIE GSKSLAQSWR MKDRMKTVSV ALVLCLNVGV DPPDVVKTTP CARLECWID PLSMGPQKAL ETIGANLQKQ YENWQPRARY KQSLDPTVDE VKKLCTSLRR NAKEERVLFH YNGHGVPRPT V NGEVWVFN ...String: MESEMLQSPL LGLGEEDEAD LTDWNLPLAF MKKRHCEKIE GSKSLAQSWR MKDRMKTVSV ALVLCLNVGV DPPDVVKTTP CARLECWID PLSMGPQKAL ETIGANLQKQ YENWQPRARY KQSLDPTVDE VKKLCTSLRR NAKEERVLFH YNGHGVPRPT V NGEVWVFN KNYTQYIPLS IYDLQTWMGS PSIFVYDCSN AGLIVKSFKQ FALQREQELE VAAINPNHPL AQMPLPPSMK NC IQLAACE ATELLPMIPD LPADLFTSCL TTPIKIALRW FCMQKCVSLV PGVTLDLIEK IPGRLNDRRT PLGELNWIFT AIT DTIAWN VLPRDLFQKL FRQDLLVASL FRNFLLAERI MRSYNCTPVS SPRLPPTYMH AMWQAWDLAV DICLSQLPTI IEEG TAFRH SPFFAEQLTA FQVWLTMGVE NRNPPEQLPI VLQVLLSQVH RLRALDLLGR FLDLGPWAVS LALSVGIFPY VLKLL QSSA RELRPLLVFI WAKILAVDSS CQADLVKDNG HKYFLSVLAD PYMPAEHRTM TAFILAVIVN SYHTGQEACL QGNLIA ICL EQLNDPHPLL RQWVAICLGR IWQNFDSARW CGVRDSAHEK LYSLLSDPIP EVRCAAVFAL GTFVGNSAER TDHSTTI DH NVAMMLAQLV SDGSPMVRKE LVVALSHLVV QYESNFCTVA LQFIEEEKNY ALPSPATTEG GSLTPVRDSP CTPRLRSV S SYGNIRAVAT ARSLNKSLQN LSLTEESGGA VAFSPGNLST SSSASSTLGS PENEEHILSF ETIDKMRRAS SYSSLNSLI GVSFNSVYTQ IWRVLLHLAA DPYPEVSDVA MKVLNSIAYK ATVNARPQRV LDTSSLTQSA PASPTNKGVH IHQAGGSPPA SSTSSSSLT NDVAKQPVSR DLPSGRPGTT GPAGAQYTPH SHQFPRTRKM FDKGPEQTAD DADDAAGHKS FISATVQTGF C DWSARYFA QPVMKIPEEH DLESQIRKER EWRFLRNSRV RRQAQQVIQK GITRLDDQIF LNRNPGVPSV VKFHPFTPCI AV ADKDSIC FWDWEKGEKL DYFHNGNPRY TRVTAMEYLN GQDCSLLLTA TDDGAIRVWK NFADLEKNPE MVTAWQGLSD MLP TTRGAG MVVDWEQETG LLMSSGDVRI VRIWDTDREM KVQDIPTGAD SCVTSLSCDS HRSLIVAGLG DGSIRVYDRR MALS ECRVM TYREHTAWVV KASLQKRPDG HIVSVSVNGD VRIFDPRMPE SVNVLQIVKG LTALDIHPQA DLIACGSVNQ FTAIY NSSG ELINNIKYYD GFMGQRVGAI SCLAFHPHWP HLAVGSNDYY ISVYSVEKRV R |
-Macromolecule #5: 1-[6-{[(3M)-4-amino-3-(2-amino-1,3-benzoxazol-5-yl)-1H-pyrazolo[3...
| Macromolecule | Name: 1-[6-{[(3M)-4-amino-3-(2-amino-1,3-benzoxazol-5-yl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]methyl}-3,4-dihydroisoquinolin-2(1H)-yl]-3-hydroxypropan-1-one type: ligand / ID: 5 / Number of copies: 1 / Formula: XZ9 |
|---|---|
| Molecular weight | Theoretical: 484.51 Da |
| Chemical component information |  ChemComp-XZ9: |
-Macromolecule #6: (3S,5R,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,30R,34aS)...
| Macromolecule | Name: (3S,5R,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,30R,34aS)-5,9,27-trihydroxy-3-{(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl- ...Name: (3S,5R,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,30R,34aS)-5,9,27-trihydroxy-3-{(2R)-1-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]propan-2-yl}-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-5,6,9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-octadecahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontine-1,11,28,29(4H,31H)-tetrone type: ligand / ID: 6 / Number of copies: 1 / Formula: XYU |
|---|---|
| Molecular weight | Theoretical: 916.188 Da |
| Chemical component information |  ChemComp-XYU: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | 2D array |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 30.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.86 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 805027 |
 Movie
Movie Controller
Controller


















 Z
Z Y
Y X
X