+ Open data
Open data
- Basic information
Basic information
| Entry | 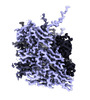 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of the CS20 bacterial adhesion pili | |||||||||
 Map data Map data | Primary map. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology | Fimbrial-type adhesion domain superfamily / Adhesion domain superfamily /  pilus / pilus /  cell adhesion / CS20 fimbria major subunit protein cell adhesion / CS20 fimbria major subunit protein Function and homology information Function and homology information | |||||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||
| Method | helical reconstruction /  cryo EM / Resolution: 3.4 Å cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Doran MH / Bullitt E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2023 Journal: Structure / Year: 2023Title: Three structural solutions for bacterial adhesion pilus stability and superelasticity. Authors: Matthew H Doran / Joseph L Baker / Tobias Dahlberg / Magnus Andersson / Esther Bullitt /   Abstract: Bacterial adhesion pili are key virulence factors that mediate host-pathogen interactions in diverse epithelial environments. Deploying a multimodal approach, we probed the structural basis ...Bacterial adhesion pili are key virulence factors that mediate host-pathogen interactions in diverse epithelial environments. Deploying a multimodal approach, we probed the structural basis underpinning the biophysical properties of pili originating from enterotoxigenic (ETEC) and uropathogenic bacteria. Using cryo-electron microscopy we solved the structures of three vaccine target pili from ETEC bacteria, CFA/I, CS17, and CS20. Pairing these and previous pilus structures with force spectroscopy and steered molecular dynamics simulations, we find a strong correlation between subunit-subunit interaction energies and the force required for pilus unwinding, irrespective of genetic similarity. Pili integrate three structural solutions for stabilizing their assemblies: layer-to-layer interactions, N-terminal interactions to distant subunits, and extended loop interactions from adjacent subunits. Tuning of these structural solutions alters the biophysical properties of pili and promotes the superelastic behavior that is essential for sustained bacterial attachment. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28152.map.gz emd_28152.map.gz | 30.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28152-v30.xml emd-28152-v30.xml emd-28152.xml emd-28152.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28152_fsc.xml emd_28152_fsc.xml | 7.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_28152.png emd_28152.png | 115.6 KB | ||
| Masks |  emd_28152_msk_1.map emd_28152_msk_1.map | 32.4 MB |  Mask map Mask map | |
| Others |  emd_28152_half_map_1.map.gz emd_28152_half_map_1.map.gz emd_28152_half_map_2.map.gz emd_28152_half_map_2.map.gz | 24.5 MB 24.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28152 http://ftp.pdbj.org/pub/emdb/structures/EMD-28152 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28152 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28152 | HTTPS FTP |
-Related structure data
| Related structure data |  8ehtMC  8ehrC  8ehsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28152.map.gz / Format: CCP4 / Size: 32.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28152.map.gz / Format: CCP4 / Size: 32.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Primary map. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.078 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_28152_msk_1.map emd_28152_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2.
| File | emd_28152_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1.
| File | emd_28152_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CS20 bacterial adhesion pili
| Entire | Name: CS20 bacterial adhesion pili |
|---|---|
| Components |
|
-Supramolecule #1: CS20 bacterial adhesion pili
| Supramolecule | Name: CS20 bacterial adhesion pili / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) / Strain: WS7179A Escherichia coli (E. coli) / Strain: WS7179A |
-Macromolecule #1: CS20 fimbria major subunit protein
| Macromolecule | Name: CS20 fimbria major subunit protein / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 17.435199 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: APAANDSSQA TLNFSGRVTS SLCQVKTDDL VKNISLGEVS KSALEATGKS PAQSFQVNLI NCDSLTDDIS YVLADANNNG TTTAYLVPK SGDTAATGVG VFVETSKGTP VNIGSDQKLD VVANKGNALS EQVIPLRAYI GTQTRAAGAI GTDVTAGTVD A TGVLTIRA ADAT |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: GOLD / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Details: 15 mA using the Pelco EasiGlow machine |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 5236 / Average electron dose: 53.62 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X


























