+ Open data
Open data
- Basic information
Basic information
| Entry | 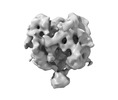 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Ligand-free Lassa GPC Trimer with C1 Symmetry | |||||||||
 Map data Map data | Sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPC / GP1 / GP2 / Lassa /  Arenavirus / Cleavage intermediate / Arenavirus / Cleavage intermediate /  VIRAL PROTEIN VIRAL PROTEIN | |||||||||
| Biological species |   Lassa virus Josiah Lassa virus Josiah | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 6.58 Å cryo EM / Resolution: 6.58 Å | |||||||||
 Authors Authors | Gorman J / Kwong PD | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Cleavage-intermediate Lassa virus trimer elicits neutralizing responses, identifies neutralizing nanobodies, and reveals an apex-situated site-of-vulnerability. Authors: Jason Gorman / Crystal Sao-Fong Cheung / Zhijian Duan / Li Ou / Maple Wang / Xuejun Chen / Cheng Cheng / Andrea Biju / Yaping Sun / Pengfei Wang / Yongping Yang / Baoshan Zhang / Jeffrey C ...Authors: Jason Gorman / Crystal Sao-Fong Cheung / Zhijian Duan / Li Ou / Maple Wang / Xuejun Chen / Cheng Cheng / Andrea Biju / Yaping Sun / Pengfei Wang / Yongping Yang / Baoshan Zhang / Jeffrey C Boyington / Tatsiana Bylund / Sam Charaf / Steven J Chen / Haijuan Du / Amy R Henry / Tracy Liu / Edward K Sarfo / Chaim A Schramm / Chen-Hsiang Shen / Tyler Stephens / I-Ting Teng / John-Paul Todd / Yaroslav Tsybovsky / Raffaello Verardi / Danyi Wang / Shuishu Wang / Zhantong Wang / Cheng-Yan Zheng / Tongqing Zhou / Daniel C Douek / John R Mascola / David D Ho / Mitchell Ho / Peter D Kwong /  Abstract: Lassa virus (LASV) infection is expanding outside its traditionally endemic areas in West Africa, posing a pandemic biothreat. LASV-neutralizing antibodies, moreover, have proven difficult to elicit. ...Lassa virus (LASV) infection is expanding outside its traditionally endemic areas in West Africa, posing a pandemic biothreat. LASV-neutralizing antibodies, moreover, have proven difficult to elicit. To gain insight into LASV neutralization, here we develop a prefusion-stabilized LASV glycoprotein trimer (GPC), pan it against phage libraries comprising single-domain antibodies (nanobodies) from shark and camel, and identify one, D5, which neutralizes LASV. Cryo-EM analyses reveal D5 to recognize a cleavage-dependent site-of-vulnerability at the trimer apex. The recognized site appears specific to GPC intermediates, with protomers lacking full cleavage between GP1 and GP2 subunits. Guinea pig immunizations with the prefusion-stabilized cleavage-intermediate LASV GPC, first as trimer and then as a nanoparticle, induce neutralizing responses, targeting multiple epitopes including that of D5; we identify a neutralizing antibody (GP23) from the immunized guinea pigs. Collectively, our findings define a prefusion-stabilized GPC trimer, reveal an apex-situated site-of-vulnerability, and demonstrate elicitation of LASV-neutralizing responses by a cleavage-intermediate LASV trimer. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26740.map.gz emd_26740.map.gz | 59.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26740-v30.xml emd-26740-v30.xml emd-26740.xml emd-26740.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_26740.png emd_26740.png | 42 KB | ||
| Masks |  emd_26740_msk_1.map emd_26740_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-26740.cif.gz emd-26740.cif.gz | 5.3 KB | ||
| Others |  emd_26740_additional_1.map.gz emd_26740_additional_1.map.gz emd_26740_half_map_1.map.gz emd_26740_half_map_1.map.gz emd_26740_half_map_2.map.gz emd_26740_half_map_2.map.gz | 30.5 MB 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26740 http://ftp.pdbj.org/pub/emdb/structures/EMD-26740 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26740 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26740 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_26740.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26740.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07325 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_26740_msk_1.map emd_26740_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map
| File | emd_26740_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_26740_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_26740_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Lassa GPC Trimer
| Entire | Name: Lassa GPC Trimer |
|---|---|
| Components |
|
-Supramolecule #1: Lassa GPC Trimer
| Supramolecule | Name: Lassa GPC Trimer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Lassa virus Josiah Lassa virus Josiah |
-Macromolecule #1: Lassa GPC Trimer
| Macromolecule | Name: Lassa GPC Trimer / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Lassa virus Josiah Lassa virus Josiah |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: TSLYKGVYEL QTLELNMETL NMTMPLSCTK NNSHHYIMVG NETGLELTLT NTSIINHKFC NLSDAHKKNL YDHALMSIIS TFHLSIPNFN QYEAMSCDFN GGKISVQYNL SHSYAGDAAN HCGTVANGVL QTFMRMAWGG SYIALDSGGC GNWDCIMTSY QYLIIQNTTW ...String: TSLYKGVYEL QTLELNMETL NMTMPLSCTK NNSHHYIMVG NETGLELTLT NTSIINHKFC NLSDAHKKNL YDHALMSIIS TFHLSIPNFN QYEAMSCDFN GGKISVQYNL SHSYAGDAAN HCGTVANGVL QTFMRMAWGG SYIALDSGGC GNWDCIMTSY QYLIIQNTTW EDHCQFSRPS PIGYLGLLSQ RTRDIYISRR RRGTFTWTLS DSEGKDTPGG YCLTRWMLIE AELKCFGNTA VAKCNEKHDE EFCDMLRLFD FNKQAIQRCK APAQMSIQLI NKAVNALIND QLIMKNHLRD IMGIPYCNYS KYWYLNHTTT GRTSLPKCWL VSNGSYLNET HFSDDIEQQA DNMITEMLQK EGGGYIPEAP RDGQAYVRKD GEWVLLSTFL GGLVPR |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: PBS |
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Pretreatment - Type: PLASMA CLEANING |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Number grids imaged: 1 / Average electron dose: 56.52 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 6.58 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 43577 |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X

































