[English] 日本語
 Yorodumi
Yorodumi- EMDB-26155: Cryo-EM structure of the human reduced folate carrier in complex ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human reduced folate carrier in complex with methotrexate | ||||||||||||
 Map data Map data | sharpened full map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords |  transporter / transporter /  reduced folate carrier / reduced folate carrier /  SLC19A1 / SLC19A1 /  TRANSPORT PROTEIN / TRANSPORT PROTEIN /  anion exchanger anion exchanger | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationfolic acid transmembrane transporter activity / folate:monoatomic anion antiporter activity / methotrexate transport / folate transmembrane transport / methotrexate transmembrane transporter activity / folic acid transport / folate import across plasma membrane / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / organic anion transport ...folic acid transmembrane transporter activity / folate:monoatomic anion antiporter activity / methotrexate transport / folate transmembrane transport / methotrexate transmembrane transporter activity / folic acid transport / folate import across plasma membrane / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / organic anion transport / 2',3'-cyclic GMP-AMP binding / xenobiotic transmembrane transport / organic anion transmembrane transporter activity / Metabolism of folate and pterines /  antiporter activity / antiporter activity /  folic acid binding / folic acid metabolic process / xenobiotic transmembrane transporter activity / transport across blood-brain barrier / female pregnancy / brush border membrane / basolateral plasma membrane / folic acid binding / folic acid metabolic process / xenobiotic transmembrane transporter activity / transport across blood-brain barrier / female pregnancy / brush border membrane / basolateral plasma membrane /  electron transfer activity / electron transfer activity /  periplasmic space / iron ion binding / apical plasma membrane / periplasmic space / iron ion binding / apical plasma membrane /  heme binding / heme binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.3 Å cryo EM / Resolution: 3.3 Å | ||||||||||||
 Authors Authors | Wright NJ / Fedor JG / Lee S-Y | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Methotrexate recognition by the human reduced folate carrier SLC19A1. Authors: Nicholas J Wright / Justin G Fedor / Han Zhang / Pyeonghwa Jeong / Yang Suo / Jiho Yoo / Jiyong Hong / Wonpil Im / Seok-Yong Lee /   Abstract: Folates are essential nutrients with important roles as cofactors in one-carbon transfer reactions, being heavily utilized in the synthesis of nucleic acids and the metabolism of amino acids during ...Folates are essential nutrients with important roles as cofactors in one-carbon transfer reactions, being heavily utilized in the synthesis of nucleic acids and the metabolism of amino acids during cell division. Mammals lack de novo folate synthesis pathways and thus rely on folate uptake from the extracellular milieu. The human reduced folate carrier (hRFC, also known as SLC19A1) is the major importer of folates into the cell, as well as chemotherapeutic agents such as methotrexate. As an anion exchanger, RFC couples the import of folates and antifolates to anion export across the cell membrane and it is a major determinant in methotrexate (antifolate) sensitivity, as genetic variants and its depletion result in drug resistance. Despite its importance, the molecular basis of substrate specificity by hRFC remains unclear. Here we present cryo-electron microscopy structures of hRFC in the apo state and captured in complex with methotrexate. Combined with molecular dynamics simulations and functional experiments, our study uncovers key determinants of hRFC transport selectivity among folates and antifolate drugs while shedding light on important features of anion recognition by hRFC. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26155.map.gz emd_26155.map.gz | 21 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26155-v30.xml emd-26155-v30.xml emd-26155.xml emd-26155.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_26155.png emd_26155.png | 57.4 KB | ||
| Filedesc metadata |  emd-26155.cif.gz emd-26155.cif.gz | 6.3 KB | ||
| Others |  emd_26155_half_map_1.map.gz emd_26155_half_map_1.map.gz emd_26155_half_map_2.map.gz emd_26155_half_map_2.map.gz | 28.3 MB 28.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26155 http://ftp.pdbj.org/pub/emdb/structures/EMD-26155 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26155 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26155 | HTTPS FTP |
-Related structure data
| Related structure data |  7tx6MC  7tx7C  8depC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26155.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26155.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened full map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map B
| File | emd_26155_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map A
| File | emd_26155_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human reduced folate carrier SLC19A1
| Entire | Name: Human reduced folate carrier SLC19A1 |
|---|---|
| Components |
|
-Supramolecule #1: Human reduced folate carrier SLC19A1
| Supramolecule | Name: Human reduced folate carrier SLC19A1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: Covalently modified Lys411 with methotrexate |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Reduced folate transporter,Soluble cytochrome b562
| Macromolecule | Name: Reduced folate transporter,Soluble cytochrome b562 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 74.797195 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MVPSSPAVEK QVPVEPGPDP ELRSWRHLVC YLCFYGFMAQ IRPGESFITP YLLGPDKNFT REQVTNEITP VLSYSYLAVL VPVFLLTDY LRYTPVLLLQ GLSFVSVWLL LLLGHSVAHM QLMELFYSVT MAARIAYSSY IFSLVRPARY QRVAGYSRAA V LLGVFTSS ...String: MVPSSPAVEK QVPVEPGPDP ELRSWRHLVC YLCFYGFMAQ IRPGESFITP YLLGPDKNFT REQVTNEITP VLSYSYLAVL VPVFLLTDY LRYTPVLLLQ GLSFVSVWLL LLLGHSVAHM QLMELFYSVT MAARIAYSSY IFSLVRPARY QRVAGYSRAA V LLGVFTSS VLGQLLVTVG RVSFSTLNYI SLAFLTFSVV LALFLKRPKR SLFFNRDLED NWETLNDNLK VIEKADNAAQ VK DALTKMR AAALDAQKAT PPKLEDKSPD SPEMKDFRHG FDILVGQIDD ALKLANEGKV KEAQAAAEQL KTTRNAYIQK YLL RVACGD SVLARMLREL GDSLRRPQLR LWSLWWVFNS AGYYLVVYYV HILWNEVDPT TNSARVYNGA ADAASTLLGA ITSF AAGFV KIRWARWSKL LIAGVTATQA GLVFLLAHTR HPSSIWLCYA AFVLFRGSYQ FLVPIATFQI ASSLSKELCA LVFGV NTFF ATIVKTIITF IVSDVRGLGL PVRKQFQLYS VYFLILSIIY FLGAMLDGLR HCQRGHHPRQ PPAQGLRSAA EEKAAQ ALS VQDKGLGGLQ PAQSPPLSPE DSLGAVGPAS LEQRQSDPYL AQAPAPQAAE FLSPVTTPSP CTLCSAQASG PEAADET CP QLAVHPPGVS KLGLQCLPSD GVQNVNQANS LEVLFQ UniProtKB: Reduced folate transporter, Soluble cytochrome b562, Reduced folate transporter |
-Macromolecule #2: METHOTREXATE
| Macromolecule | Name: METHOTREXATE / type: ligand / ID: 2 / Number of copies: 1 / Formula: MTX |
|---|---|
| Molecular weight | Theoretical: 454.439 Da |
| Chemical component information | 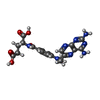 ChemComp-MTX: |
-Macromolecule #3: Digitonin
| Macromolecule | Name: Digitonin / type: ligand / ID: 3 / Number of copies: 1 / Formula: AJP |
|---|---|
| Molecular weight | Theoretical: 1.229312 KDa |
| Chemical component information |  ChemComp-AJP: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 200 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.00039000000000000005 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 277 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 5 / Number real images: 25507 / Average exposure time: 4.6 sec. / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 9098387 |
|---|---|
| Startup model | Type of model: NONE |
| Initial angle assignment | Type: PROJECTION MATCHING / Software - Name: RELION (ver. 3.1) |
| Final angle assignment | Type: PROJECTION MATCHING / Software - Name: cryoSPARC (ver. 3) |
| Final reconstruction | Number classes used: 1 / Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 3) / Number images used: 492117 |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 53.6 |
|---|---|
| Output model |  PDB-7tx6: |
 Movie
Movie Controller
Controller















 Z
Z Y
Y X
X

















