[English] 日本語
 Yorodumi
Yorodumi- EMDB-25178: Sub-tomogram averaged structure of HIV-1 Envelope protein in nati... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25178 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Sub-tomogram averaged structure of HIV-1 Envelope protein in native membrane | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
| Function / homology |  Function and homology information Function and homology informationvirus-mediated perturbation of host defense response => GO:0019049 / : / stimulatory C-type lectin receptor signaling pathway / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / membrane => GO:0016020 /  viral protein processing ...virus-mediated perturbation of host defense response => GO:0019049 / : / stimulatory C-type lectin receptor signaling pathway / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / membrane => GO:0016020 / viral protein processing ...virus-mediated perturbation of host defense response => GO:0019049 / : / stimulatory C-type lectin receptor signaling pathway / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / membrane => GO:0016020 /  viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||
| Biological species |    Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||||||||
| Method | subtomogram averaging /  cryo EM / Resolution: 9.1 Å cryo EM / Resolution: 9.1 Å | ||||||||||||
 Authors Authors | Mangala Prasad V / Lee KK | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Cryo-ET of Env on intact HIV virions reveals structural variation and positioning on the Gag lattice. Authors: Vidya Mangala Prasad / Daniel P Leaman / Klaus N Lovendahl / Jacob T Croft / Mark A Benhaim / Edgar A Hodge / Michael B Zwick / Kelly K Lee /  Abstract: HIV-1 Env mediates viral entry into host cells and is the sole target for neutralizing antibodies. However, Env structure and organization in its native virion context has eluded detailed ...HIV-1 Env mediates viral entry into host cells and is the sole target for neutralizing antibodies. However, Env structure and organization in its native virion context has eluded detailed characterization. Here, we used cryo-electron tomography to analyze Env in mature and immature HIV-1 particles. Immature particles showed distinct Env positioning relative to the underlying Gag lattice, providing insights into long-standing questions about Env incorporation. A 9.1-Å sub-tomogram-averaged reconstruction of virion-bound Env in conjunction with structural mass spectrometry revealed unexpected features, including a variable central core of the gp41 subunit, heterogeneous glycosylation between protomers, and a flexible stalk that allows Env tilting and variable exposure of neutralizing epitopes. Together, our results provide an integrative understanding of HIV assembly and structural variation in Env antigen presentation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25178.map.gz emd_25178.map.gz | 855.6 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25178-v30.xml emd-25178-v30.xml emd-25178.xml emd-25178.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25178_fsc.xml emd_25178_fsc.xml | 4.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_25178.png emd_25178.png | 57.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25178 http://ftp.pdbj.org/pub/emdb/structures/EMD-25178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25178 | HTTPS FTP |
-Related structure data
| Related structure data |  7skaMC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25178.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25178.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 2.58 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human immunodeficiency virus 1
| Entire | Name:    Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
|---|---|
| Components |
|
-Supramolecule #1: Human immunodeficiency virus 1
| Supramolecule | Name: Human immunodeficiency virus 1 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 / Details: Env glycoprotein displayed on surface of HIV-1 / NCBI-ID: 11676 / Sci species name: Human immunodeficiency virus 1 / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host system | Organism:   Homo sapiens (human) / Recombinant cell: HEK293T Homo sapiens (human) / Recombinant cell: HEK293T |
| Molecular weight | Theoretical: 430 KDa |
-Macromolecule #1: Envelope glycoprotein gp120
| Macromolecule | Name: Envelope glycoprotein gp120 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 52.170992 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: KLWVTVYYGV PVWKEATTTL FCASDAKAYD TEVHNVWATH ACVPTDPNPQ EVVLENVTEN FNMWKNNMVE QMHEDIISLW DQSLKPCVK LTPLCVTLNC TDLRNVTNIN NSSEGMRGEI KNCSFNITTS IRDKVKKDYA LFYRLDVVPI DNDNTSYRLI N CNTSTITQ ...String: KLWVTVYYGV PVWKEATTTL FCASDAKAYD TEVHNVWATH ACVPTDPNPQ EVVLENVTEN FNMWKNNMVE QMHEDIISLW DQSLKPCVK LTPLCVTLNC TDLRNVTNIN NSSEGMRGEI KNCSFNITTS IRDKVKKDYA LFYRLDVVPI DNDNTSYRLI N CNTSTITQ ACPKVSFEPI PIHYCTPAGF AILKCKDKKF NGTGPCKNVS TVQCTHGIRP VVSTQLLLNG SLAEEEVVIR SS NFTDNAK NIIVQLKESV EINCTRPNNN TRKSIHIGPG RAFYTTGEII GDIRQAHCNI SRTKWNNTLN QIATKLKEQF GNN KTIVFN QSSGGDPEIV MHSFNCGGEF FYCNSTQLFN STWNFNGTWN LTQSNGTEGN DTITLPCRIK QIINMWQEVG KAMY APPIR GQIRCSSNIT GLILTRDGGT NSSGSEIFRP GGGDMRDNWR SELYKYKVVK IEPLGVAPTK |
-Macromolecule #2: Envelope glycoprotein gp41
| Macromolecule | Name: Envelope glycoprotein gp41 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:    Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 16.420559 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GFLGAAGSTM GAASMTLTVQ ARQLLSGIVQ QQNNLLRAIE AQQHLLQLTV WGIRQLQARV LAVERYLRDQ QLLGIWGCSG KLICTTAVP WNASWSNKSL EQIWNNMTWM EWDREINNYT SLIHSLIEEA QNQQEKNEQE LLELD |
-Macromolecule #12: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 12 / Number of copies: 21 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #13: alpha-L-fucopyranose
| Macromolecule | Name: alpha-L-fucopyranose / type: ligand / ID: 13 / Number of copies: 3 / Formula: FUC |
|---|---|
| Molecular weight | Theoretical: 164.156 Da |
| Chemical component information | 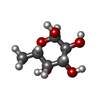 ChemComp-FUC: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Component - Concentration: 1.0 X / Component - Name: Phosphate buffer saline |
|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 2.5 µm / Nominal magnification: 58000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 2.5 µm / Nominal magnification: 58000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 2.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















