[English] 日本語
 Yorodumi
Yorodumi- EMDB-22381: Structure of the activated Roq1 resistosome directly recognizing ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22381 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
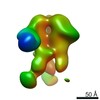
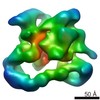
| Title | Structure of the activated Roq1 resistosome directly recognizing the pathogen effector XopQ | |||||||||
 Map data Map data | Structure of the activated Roq1 resistosome directly recognizing the pathogen effector XopQ | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Resistosome /  Plant Immunity / Effector / LRR / TIR / NB-ARC / Plant Immunity / Effector / LRR / TIR / NB-ARC /  IMMUNE SYSTEM IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NAD+ nucleotidase, cyclic ADP-ribose generating / NADP+ nucleosidase activity /  Hydrolases; Glycosylases; Hydrolysing N-glycosyl compounds / Hydrolases; Glycosylases; Hydrolysing N-glycosyl compounds /  ADP binding / defense response / ADP binding / defense response /  signal transduction signal transductionSimilarity search - Function | |||||||||
| Biological species |   Nicotiana benthamiana (plant) / Nicotiana benthamiana (plant) /   Xanthomonas euvesicatoria (bacteria) Xanthomonas euvesicatoria (bacteria) | |||||||||
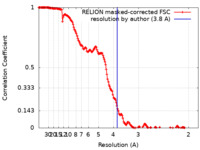
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.8 Å cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Martin R / Qi T | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2020 Journal: Science / Year: 2020Title: Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Authors: Raoul Martin / Tiancong Qi / Haibo Zhang / Furong Liu / Miles King / Claire Toth / Eva Nogales / Brian J Staskawicz /   Abstract: Plants and animals detect pathogen infection using intracellular nucleotide-binding leucine-rich repeat receptors (NLRs) that directly or indirectly recognize pathogen effectors and activate an ...Plants and animals detect pathogen infection using intracellular nucleotide-binding leucine-rich repeat receptors (NLRs) that directly or indirectly recognize pathogen effectors and activate an immune response. How effector sensing triggers NLR activation remains poorly understood. Here we describe the 3.8-angstrom-resolution cryo-electron microscopy structure of the activated ROQ1 (recognition of XopQ 1), an NLR native to with a Toll-like interleukin-1 receptor (TIR) domain bound to the effector XopQ ( outer protein Q). ROQ1 directly binds to both the predicted active site and surface residues of XopQ while forming a tetrameric resistosome that brings together the TIR domains for downstream immune signaling. Our results suggest a mechanism for the direct recognition of effectors by NLRs leading to the oligomerization-dependent activation of a plant resistosome and signaling by the TIR domain. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22381.map.gz emd_22381.map.gz | 395.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22381-v30.xml emd-22381-v30.xml emd-22381.xml emd-22381.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22381_fsc.xml emd_22381_fsc.xml | 17.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_22381.png emd_22381.png | 105.5 KB | ||
| Filedesc metadata |  emd-22381.cif.gz emd-22381.cif.gz | 6.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22381 http://ftp.pdbj.org/pub/emdb/structures/EMD-22381 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22381 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22381 | HTTPS FTP |
-Related structure data
| Related structure data |  7jlvMC  7jluC  7jlxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22381.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22381.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of the activated Roq1 resistosome directly recognizing the pathogen effector XopQ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9386 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Roq1
| Entire | Name: Roq1 |
|---|---|
| Components |
|
-Supramolecule #1: Roq1
| Supramolecule | Name: Roq1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Nicotiana benthamiana (plant) Nicotiana benthamiana (plant) |
| Molecular weight | Theoretical: 49.819 KDa |
-Supramolecule #2: XopQ
| Supramolecule | Name: XopQ / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Xanthomonas euvesicatoria (bacteria) Xanthomonas euvesicatoria (bacteria) |
-Macromolecule #1: Disease resistance protein Roq1
| Macromolecule | Name: Disease resistance protein Roq1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase |
|---|---|
| Source (natural) | Organism:   Nicotiana benthamiana (plant) Nicotiana benthamiana (plant) |
| Molecular weight | Theoretical: 150.648391 KDa |
| Recombinant expression | Organism:   Nicotiana benthamiana (plant) Nicotiana benthamiana (plant) |
| Sequence | String: MLTSSSHHGR SYDVFLSFRG EDTRKTFVGH LFNALIEKGI HTFMDDKELK RGKSISSELM KAIGESRFAV VVFSKNYASS TWCLEELVK ILEIHEKFEL IVVPVFYDVD PSTVRKQNGE YAVCFTKFEA NLVDDRDKVL RWREALTKVA NISGHDLRNT Y NGDESKCI ...String: MLTSSSHHGR SYDVFLSFRG EDTRKTFVGH LFNALIEKGI HTFMDDKELK RGKSISSELM KAIGESRFAV VVFSKNYASS TWCLEELVK ILEIHEKFEL IVVPVFYDVD PSTVRKQNGE YAVCFTKFEA NLVDDRDKVL RWREALTKVA NISGHDLRNT Y NGDESKCI QQILKDIFDK FCFSISITNR DLVGIESQIK KLSSLLRMDL KGVRLVGIWG MGGVGKTTAA RALFNRYYQN FE SACFLED VKEYLQHHTL LYLQKTLLSK LLKVEFVDCT DTEEMCVILK RRLCSKKVLV VLDDVNHNDQ LDKLVGAEDW FGS GSRIVI TTRDMKLLKN HDVHETYEIK VLEKDEAIEL FNLHAFKRSS PEKEFKELLN LVVDYTGGLP LALKVLGSLL YKED LDVWI STIDRLKDNP EGEIMATLKI SFDGLRDYEK SIFLDIACFF RGYNQRDMTA LFHASGFHPV LGVKTLVEKS LIFIL EDKI QMHDLMQEMG RQIAVQESPM RRIYRPEDVK DACIGDMRKE AIEGLLLTEP EQFEEGELEY MYSAEALKKT RRLRIL VKE YYNRGFDEPV AYLPNSLLWL EWRNYSSNSF PSNFEPSKLV YLTMKGSSII ELWNGAKRLA FLTTLDLSYC HKLIQTP DF RMITNLERLI LSSCDALVEV HPSVGFLKNL ILLNMDHCIS LERLPAIIQS ECLEVLDLNY CFNLKMFPEV ERNMTHLK K LDLTSTGIRE LPASIEHLSS LENLQMHSCN QLVSLPSSIW RFRNLKISEC EKLGSLPEIH GNSNCTRELI LKLVSIKEL PTSIGNLTSL NFLEICNCKT ISSLSSSIWG LTSLTTLKLL DCRKLKNLPG IPNAINHLSG HGLQLLLTLE QPTIYERLDL LRIIDMSWC SCISSLPHNI WMLKFLRILC ISYCSRLEYL PENLGHLEHL EELLADGTGI LRLPSSVARL NKLEVLSFRK K FAIGPKVQ YSSSMLNLPD DVFGSLGSLG SVVKLNLSGN GFCNLPETMN QLFCLEYLDI TFCQRLEALP ELPPSIKELY VD EHLALRI MEDLVIKCKE LNLIAVTKIE YQNFYRWLDS IWSDVSELLE NSQKQQLDDM LQLIPFSYLS TAKREEVLKI VIH GTRIPE WFRWQDRSAT TMSVNLPEYW YTENFLGFAI CCSCCFYHSA RSYDVEFEGS MHHYNYDSSY WKEYEEPSYD FYER DSIEI TAKLTPRHKG MRTEELKKVC SFSMNVLRRA TAVPNMCFAF FPFNSLCHIS NLQANNPNDY GIFETCLSPG DIRHR GKQW GFNLVYKDET GGSVTHEMLI NR UniProtKB:  Disease resistance protein Roq1 Disease resistance protein Roq1 |
-Macromolecule #2: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 4 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: PLASMA CLEANING | ||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: 10 sec blot. Blot Force 10. 90 min incubation.. | ||||||||||||||
| Details | Sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 80879 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: -2.5 µm / Nominal defocus min: -0.9 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: -2.5 µm / Nominal defocus min: -0.9 µm |
| Specialist optics | Energy filter - Name: GIF Bioquantum |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 11134 / Average electron dose: 50.0 e/Å2 Details: Images were collected as dose-fractionated movie frames. |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller