[English] 日本語
 Yorodumi
Yorodumi- EMDB-18394: Focused map of Hantaan virus polymerase dimer used to refine the ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Focused map of Hantaan virus polymerase dimer used to refine the cap-binding domain | |||||||||
 Map data Map data | HTNV-L Apo Dimer focused on CBD | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Bunyavirus / Hantaan virus / Bunyavirus / Hantaan virus /  polymerase / dimer / polymerase / dimer /  VIRAL PROTEIN VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRNA-templated viral transcription / negative stranded viral RNA replication /  cap snatching / cap snatching /  endonuclease activity / endonuclease activity /  Hydrolases; Acting on ester bonds / host cell perinuclear region of cytoplasm / Hydrolases; Acting on ester bonds / host cell perinuclear region of cytoplasm /  RNA-directed RNA polymerase / RNA-directed RNA polymerase /  RNA-dependent RNA polymerase activity / RNA-dependent RNA polymerase activity /  nucleotide binding / DNA-templated transcription / nucleotide binding / DNA-templated transcription /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||
| Biological species |   Hantaan virus 76-118 Hantaan virus 76-118 | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Durieux Trouilleton Q / Arragain B / Malet H | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural characterization of the oligomerization of full-length Hantaan virus polymerase into symmetric dimers and hexamers. Authors: Quentin Durieux Trouilleton / Dominique Housset / Paco Tarillon / Benoît Arragain / Hélène Malet /  Abstract: Hantaan virus is a dangerous human pathogen whose segmented negative-stranded RNA genome is replicated and transcribed by a virally-encoded multi-functional polymerase. Here we describe the complete ...Hantaan virus is a dangerous human pathogen whose segmented negative-stranded RNA genome is replicated and transcribed by a virally-encoded multi-functional polymerase. Here we describe the complete cryo-electron microscopy structure of Hantaan virus polymerase in several oligomeric forms. Apo polymerase protomers can adopt two drastically different conformations, which assemble into two distinct symmetric homodimers, that can themselves gather to form hexamers. Polymerase dimerization induces the stabilization of most polymerase domains, including the C-terminal domain that contributes the most to dimer's interface, along with a lariat region that participates to the polymerase steadying. Binding to viral RNA induces significant conformational changes resulting in symmetric oligomer disruption and polymerase activation, suggesting the possible involvement of apo multimers as protecting systems that would stabilize the otherwise flexible C-terminal domains. Overall, these results provide insights into the multimerization capability of Hantavirus polymerase and may help to define antiviral compounds to counteract these life-threatening viruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18394.map.gz emd_18394.map.gz | 4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18394-v30.xml emd-18394-v30.xml emd-18394.xml emd-18394.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18394_fsc.xml emd_18394_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_18394.png emd_18394.png | 50.9 KB | ||
| Filedesc metadata |  emd-18394.cif.gz emd-18394.cif.gz | 6.9 KB | ||
| Others |  emd_18394_half_map_1.map.gz emd_18394_half_map_1.map.gz emd_18394_half_map_2.map.gz emd_18394_half_map_2.map.gz | 247.7 MB 247.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18394 http://ftp.pdbj.org/pub/emdb/structures/EMD-18394 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18394 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18394 | HTTPS FTP |
-Related structure data
| Related structure data |  8qe5C  8qgtC  8qguC  8qh3C  8qhdC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_18394.map.gz / Format: CCP4 / Size: 266.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18394.map.gz / Format: CCP4 / Size: 266.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HTNV-L Apo Dimer focused on CBD | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.839 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: HTNV-L Apo Dimer focused on CBD half map A
| File | emd_18394_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HTNV-L Apo Dimer focused on CBD half map A | ||||||||||||
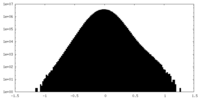
| Projections & Slices |
| ||||||||||||
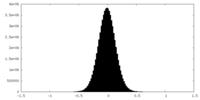
| Density Histograms |
-Half map: HTNV-L Apo Dimer focused on CBD half map B
| File | emd_18394_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HTNV-L Apo Dimer focused on CBD half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
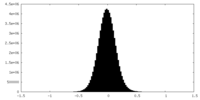
| Density Histograms |
- Sample components
Sample components
-Entire : Hantaan virus polymerase
| Entire | Name: Hantaan virus polymerase |
|---|---|
| Components |
|
-Supramolecule #1: Hantaan virus polymerase
| Supramolecule | Name: Hantaan virus polymerase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Hantaan virus 76-118 Hantaan virus 76-118 |
| Molecular weight | Theoretical: 500 KDa |
-Macromolecule #1: Hantaan virus polymerase
| Macromolecule | Name: Hantaan virus polymerase / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Hantaan virus 76-118 Hantaan virus 76-118 |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGHHHHHHDY DIPTTENLYF QGMDKYREIH NKLKEFSPGT LTAVECIDYL DRLYAVRHDI VDQMIKHDWS DNKDSEEAIG KVLLFAGVPS NIITALEKKI IPNHPTGKSL KAFFKMTPDN YKISGTTIEF VEVTVTADVD KGIREKKLKY EAGLTYIEQE LHKFFLKGEI ...String: MGHHHHHHDY DIPTTENLYF QGMDKYREIH NKLKEFSPGT LTAVECIDYL DRLYAVRHDI VDQMIKHDWS DNKDSEEAIG KVLLFAGVPS NIITALEKKI IPNHPTGKSL KAFFKMTPDN YKISGTTIEF VEVTVTADVD KGIREKKLKY EAGLTYIEQE LHKFFLKGEI PQPYKITFNV VAVRTDGSNI TTQWPSRRND GVVQYMRLVQ AEISYVREHL IKTEERAALE AMFNLKFNIS THKSQPYYIP DYKGMEPIGA NIEDLVDYSK DWLSRARNFS FFEVKGTAVF ECFNSNEANH CQRYPMSRKP RNFLLIQCSL ITSYKPATTL SDQIDSRRAC SYILNLIPDT PASYLIHDMA YRYINLTRED MINYYAPRIQ FKQTQNVREP GTFKLTSSML RAESKAMLDL LNNHKSGEKH GAQIESLNIA SHIVQSESVS LITKILSDLE LNITEPSTQE YSTTKHTYVD TVLDKFFQNE TQKYLIDVLK KTTAWHIGHL IRDITESLIA HSGLKRSKYW SLHSYNNGNV ILFILPSKSL EVAGSFIRFI TVFRIGPGLV DKDNLDTILI DGDSQWGVSK VMSIDLNRLL ALNIAFEKAL IATATWFQYY TEDQGQFPLQ YAIRSVFANH FLLAICQKMK LCAIFDNLRY LIPAVTSLYS GFPSLIEKLF ERPFKSSLEV YIYYNIKSLL VALAQNNKAR FYSKVKLLGL TVDQSTVGAS GVYPSFMSRI VYKHYRSLIS EVTTCFFLFE KGLHGNMNEE AKIHLETVEW ALKFREKEEK YGESLVENGY MMWELRANAE LAEQQLYCQD AIELAAIELN KVLATKSSVV ANSILSKNWE EPYFSQTRNI SLKGMSGQVQ EDGHLSSSVT IIEAIRYLSN SRHNPSLLKL YEETREQKAM ARIVRKYQRT EADRGFFITT LPTRCRLEII EDYYDAIAKN ISEEYISYGG EKKILAIQGA LEKALRWASG ESFIELSNHK FIRMKRKLMY VSADATKWSP GDNSAKFRRF TSMLHNGLPN NKLKNCVIDA LKQVYKTDFF MSRKLRNYID SMESLDPHIK QFLDFFPDGH HGEVKGNWLQ GNLNKCSSLF GVAMSLLFKQ VWTNLFPELD CFFEFAHHSD DALFIYGYLE PVDDGTDWFL FVSQQIQAGH LHWFSVNTEM WKSMFNLHEH ILLLGSIKIS PKKTTVSPTN AEFLSTFFEG CAVSIPFVKI LLGSLSDLPG LGYFDDLAAA QSRCVKALDL GASPQVAQLA VALCTSKVER LYGTAPGMVN HPAAYLQVKH TDTPIPLGGN GAMSIMELAT AGIGMSDKNL LKRALLGYSH KRQKSMLYIL GLFKFLMKLS DETFQHERLG QFSFIGKVQW KIFTPKSEFE FADMYTSKFL ELWSSQHVTY DYIIPKGRDN LLIYLVRKLN DPSIVTAMTM QSPLQLRFRM QAKQHMKVCR LDGEWVTFRE VLAAANSFAE NYSATSQDMD LFQTLTSCTF SKEYAWKDFL NGIHCDVIPT KQVQRAKVAR TFTVREKDQI IQNSIPAVIG YKFAVTVEEM SDVLDTAKFP DSLSVDLKTM KDGVYRELGL DISLPDVMKR IAPMLYKSSK SRVVIVQGNV EGTAEAICRY WLKSMSLVKT IRVKPHKEVL QAVSIFNRKE DIGQQKDLAA LKLCIEVWRW CKANSAPYRD WFQALWFEDK TFSEWLDRFC RVGVPPIDPE IQCAALMIAD IKGDYSVLQL QANRRAYSGK QYDAYCVQTY NEVTKLYEGD LRVTFNFGLD CARLEIFWDK KAYILETSIT QKHVLKIMMD EVSKELIKCG MRFNTEQVQG VRHMVLFKTE SGFEWGKPNI PCIVYKNCVL RTSLRTTQAI NHKFMITIKD DGLRAIAQHD EDSPRFLLAH AFHTIRDIRY QAVDAVSNVW FIHKGVKLYL NPIISSGLLE NFMKNLPAAI PPAAYSLIMN RAKISVDLFM FNDLLKLINP RNTLDLSGLE TTGDEFSTVS SMSSRLWSEE MSLVDDDEEL DDEFTIDLQD VDFENIDIEA DIEHFLQDES SYTGDLLIST EETESKKMRG IVKILEPVRL IKSWVSRGLS IEKVYSPVNI ILMSRYISKT FNLSTKQVSL LDPYDLTELE SIVRGWGECV IDQFESLDRE AQNMVVNKGI CPEDVIPDSL FSFRHTMVLL RRLFPQDSIS SFY UniProtKB: RNA-directed RNA polymerase L |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.25 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 50 sec. / Pretreatment - Atmosphere: AIR / Details: 25 mA | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 77 K / Instrument: FEI VITROBOT MARK IV / Details: blot force 1 3s. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.0 µm / Calibrated defocus min: 0.8 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 14650 / Average exposure time: 1.45 sec. / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X