+ Open data
Open data
- Basic information
Basic information
| Entry | 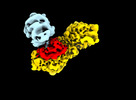 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | MTHFR + SAH asymmetric dis-inhibited state | |||||||||
 Map data Map data | Main local filtered | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Dis-inhibited /  allosteric / allosteric /  folate / folate /  S-adenosylhomocysteine / S-adenosylhomocysteine /  FLAVOPROTEIN FLAVOPROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmethylenetetrahydrofolate reductase (NADPH) / response to vitamin B2 / methylenetetrahydrofolate reductase (NADPH) activity /  methylenetetrahydrofolate reductase (NAD(P)H) activity / methionine metabolic process / modified amino acid binding / homocysteine metabolic process / heterochromatin organization / S-adenosylmethionine metabolic process / methionine biosynthetic process ...methylenetetrahydrofolate reductase (NADPH) / response to vitamin B2 / methylenetetrahydrofolate reductase (NADPH) activity / methylenetetrahydrofolate reductase (NAD(P)H) activity / methionine metabolic process / modified amino acid binding / homocysteine metabolic process / heterochromatin organization / S-adenosylmethionine metabolic process / methionine biosynthetic process ...methylenetetrahydrofolate reductase (NADPH) / response to vitamin B2 / methylenetetrahydrofolate reductase (NADPH) activity /  methylenetetrahydrofolate reductase (NAD(P)H) activity / methionine metabolic process / modified amino acid binding / homocysteine metabolic process / heterochromatin organization / S-adenosylmethionine metabolic process / methionine biosynthetic process / Metabolism of folate and pterines / response to folic acid / tetrahydrofolate interconversion / response to amino acid / FAD binding / response to interleukin-1 / neural tube closure / methylenetetrahydrofolate reductase (NAD(P)H) activity / methionine metabolic process / modified amino acid binding / homocysteine metabolic process / heterochromatin organization / S-adenosylmethionine metabolic process / methionine biosynthetic process / Metabolism of folate and pterines / response to folic acid / tetrahydrofolate interconversion / response to amino acid / FAD binding / response to interleukin-1 / neural tube closure /  flavin adenine dinucleotide binding / flavin adenine dinucleotide binding /  NADP binding / response to hypoxia / response to xenobiotic stimulus / protein-containing complex binding / NADP binding / response to hypoxia / response to xenobiotic stimulus / protein-containing complex binding /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.14 Å cryo EM / Resolution: 3.14 Å | |||||||||
 Authors Authors | Blomgren LKM / Yue WW / Froese DS / McCorvie TJ | |||||||||
| Funding support |  Switzerland, 1 items Switzerland, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Dynamic inter-domain transformations mediate the allosteric regulation of human 5, 10-methylenetetrahydrofolate reductase. Authors: Linnea K M Blomgren / Melanie Huber / Sabrina R Mackinnon / Céline Bürer / Arnaud Baslé / Wyatt W Yue / D Sean Froese / Thomas J McCorvie /   Abstract: 5,10-methylenetetrahydrofolate reductase (MTHFR) commits folate-derived one-carbon units to generate the methyl-donor S-adenosyl-L-methionine (SAM). Eukaryotic MTHFR appends to the well-conserved ...5,10-methylenetetrahydrofolate reductase (MTHFR) commits folate-derived one-carbon units to generate the methyl-donor S-adenosyl-L-methionine (SAM). Eukaryotic MTHFR appends to the well-conserved catalytic domain (CD) a unique regulatory domain (RD) that confers feedback inhibition by SAM. Here we determine the cryo-electron microscopy structures of human MTHFR bound to SAM and its demethylated product S-adenosyl-L-homocysteine (SAH). In the active state, with the RD bound to a single SAH, the CD is flexible and exposes its active site for catalysis. However, in the inhibited state the RD pocket is remodelled, exposing a second SAM-binding site that was previously occluded. Dual-SAM bound MTHFR demonstrates a substantially rearranged inter-domain linker that reorients the CD, inserts a loop into the active site, positions Tyr404 to bind the cofactor FAD, and blocks substrate access. Our data therefore explain the long-distance regulatory mechanism of MTHFR inhibition, underpinned by the transition between dual-SAM and single-SAH binding in response to cellular methylation status. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18299.map.gz emd_18299.map.gz | 5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18299-v30.xml emd-18299-v30.xml emd-18299.xml emd-18299.xml | 22.8 KB 22.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18299_fsc.xml emd_18299_fsc.xml | 13.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_18299.png emd_18299.png | 46.3 KB | ||
| Masks |  emd_18299_msk_1.map emd_18299_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-18299.cif.gz emd-18299.cif.gz | 6.7 KB | ||
| Others |  emd_18299_additional_1.map.gz emd_18299_additional_1.map.gz emd_18299_additional_2.map.gz emd_18299_additional_2.map.gz emd_18299_half_map_1.map.gz emd_18299_half_map_1.map.gz emd_18299_half_map_2.map.gz emd_18299_half_map_2.map.gz | 230 MB 120.6 MB 226.5 MB 226.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18299 http://ftp.pdbj.org/pub/emdb/structures/EMD-18299 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18299 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18299 | HTTPS FTP |
-Related structure data
| Related structure data |  8qa5MC  8qa4C  8qa6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18299.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18299.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main local filtered | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.574 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_18299_msk_1.map emd_18299_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened
| File | emd_18299_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Non-sharpened
| File | emd_18299_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Non-sharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_18299_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_18299_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human 5,10-methylenetetrahydrofolate reductase in complex with S-...
| Entire | Name: Human 5,10-methylenetetrahydrofolate reductase in complex with S-Adenosyl-L-homocysteine, regulatory domains with one catalytic domain |
|---|---|
| Components |
|
-Supramolecule #1: Human 5,10-methylenetetrahydrofolate reductase in complex with S-...
| Supramolecule | Name: Human 5,10-methylenetetrahydrofolate reductase in complex with S-Adenosyl-L-homocysteine, regulatory domains with one catalytic domain type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 150 KDa |
-Macromolecule #1: Methylenetetrahydrofolate reductase (NADPH)
| Macromolecule | Name: Methylenetetrahydrofolate reductase (NADPH) / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 75.461195 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MVNEARGNSS LNPCLEGSAS SGSESSKDSS RCSTPGLDPE RHERLREKMR RRLESGDKWF SLEFFPPRTA EGAVNLISRF DRMAAGGPL YIDVTWHPAG DPGSDKETSS MMIASTAVNY CGLETILHMT CCRQRLEEIT GHLHKAKQLG LKNIMALRGD P IGDQWEEE ...String: MVNEARGNSS LNPCLEGSAS SGSESSKDSS RCSTPGLDPE RHERLREKMR RRLESGDKWF SLEFFPPRTA EGAVNLISRF DRMAAGGPL YIDVTWHPAG DPGSDKETSS MMIASTAVNY CGLETILHMT CCRQRLEEIT GHLHKAKQLG LKNIMALRGD P IGDQWEEE EGGFNYAVDL VKHIRSEFGD YFDICVAGYP KGHPEAGSFE ADLKHLKEKV SAGADFIITQ LFFEADTFFR FV KACTDMG ITCPIVPGIF PIQGYHSLRQ LVKLSKLEVP QEIKDVIEPI KDNDAAIRNY GIELAVSLCQ ELLASGLVPG LHF YTLNRE MATTEVLKRL GMWTEDPRRP LPWALSAHPK RREEDVRPIF WASRPKSYIY RTQEWDEFPN GRWGNSSSPA FGEL KDYYL FYLKSKSPKE ELLKMWGEEL TSEASVFEVF VLYLSGEPNR NGHKVTCLPW NDEPLAAETS LLKEELLRVN RQGIL TINS QPNINGKPSS DPIVGWGPSG GYVFQKAYLE FFTSRETAEA LLQVLKKYEL RVNYHLVNVK GENITNAPEL QPNAVT WGI FPGREIIQPT VVDPVSFMFW KDEAFALWIE QWGKLYEEES PSRTIIQYIH DNYFLVNLVD NDFPLDNCLW QVVEDTL EL LNRPTQNARE TEAPAENLYF Q UniProtKB: Methylenetetrahydrofolate reductase (NADPH) |
-Macromolecule #2: FLAVIN-ADENINE DINUCLEOTIDE
| Macromolecule | Name: FLAVIN-ADENINE DINUCLEOTIDE / type: ligand / ID: 2 / Number of copies: 1 / Formula: FAD |
|---|---|
| Molecular weight | Theoretical: 785.55 Da |
| Chemical component information |  ChemComp-FAD: |
-Macromolecule #3: S-ADENOSYL-L-HOMOCYSTEINE
| Macromolecule | Name: S-ADENOSYL-L-HOMOCYSTEINE / type: ligand / ID: 3 / Number of copies: 2 / Formula: SAH |
|---|---|
| Molecular weight | Theoretical: 384.411 Da |
| Chemical component information |  ChemComp-SAH: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 20 mM HEPES, pH 7.5, 150 mM NaCl, 0.0025% Tween20, 1 mM S-Adenosyl-L-homocysteine, filter sterilised |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 40 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 240000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 240000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 2 / Number real images: 5606 / Average exposure time: 5.18 sec. / Average electron dose: 50.0 e/Å2 |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 86.3 / Target criteria: Cross-correlation coeficient |
| Output model |  PDB-8qa5: |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X