+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | OMI-42 FAB IN COMPLEX WITH SARS-COV-2 BETA SPIKE GLYCOPROTEIN | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords |  SARS-COV2 / SPIKE / SARS-COV2 / SPIKE /  GLYCOPROTEIN / GLYCOPROTEIN /  ANTIBODY / ANTIBODY /  FAB / B.1.135 / FAB / B.1.135 /  BETA VARIANT / BETA VARIANT /  COMPLEX / NEUTRALISING / CONVALESCENT SERA / COMPLEX / NEUTRALISING / CONVALESCENT SERA /  VIRAL PROTEIN/IMMUNE SYSTEM / VIRAL PROTEIN/IMMUNE SYSTEM /  VIRAL PROTEIN / VIRAL PROTEIN /  IMMUNE SYSTEM COMPLEX / IMMUNE SYSTEM COMPLEX /  STRUCTURAL PROTEIN STRUCTURAL PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology information virion component / Maturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ... virion component / Maturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ... virion component / Maturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / entry receptor-mediated virion attachment to host cell / receptor-mediated endocytosis of virus by host cell / Attachment and Entry / virion component / Maturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / entry receptor-mediated virion attachment to host cell / receptor-mediated endocytosis of virus by host cell / Attachment and Entry /  membrane fusion / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / membrane fusion / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell /  receptor ligand activity / host cell surface receptor binding / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / receptor ligand activity / host cell surface receptor binding / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / viral envelope / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||
| Biological species |   Severe acute respiratory syndrome coronavirus 2 / Severe acute respiratory syndrome coronavirus 2 /   Homo sapiens (human) / Homo sapiens (human) /   Tequatrovirus T4 Tequatrovirus T4 | ||||||||||||
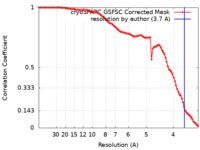
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.7 Å cryo EM / Resolution: 3.7 Å | ||||||||||||
 Authors Authors | Duyvesteyn HME / Ren J / Stuart DI | ||||||||||||
| Funding support |  China, China,  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Potent cross-reactive antibodies following Omicron breakthrough in vaccinees. Authors: Rungtiwa Nutalai / Daming Zhou / Aekkachai Tuekprakhon / Helen M Ginn / Piyada Supasa / Chang Liu / Jiandong Huo / Alexander J Mentzer / Helen M E Duyvesteyn / Aiste Dijokaite-Guraliuc / ...Authors: Rungtiwa Nutalai / Daming Zhou / Aekkachai Tuekprakhon / Helen M Ginn / Piyada Supasa / Chang Liu / Jiandong Huo / Alexander J Mentzer / Helen M E Duyvesteyn / Aiste Dijokaite-Guraliuc / Donal Skelly / Thomas G Ritter / Ali Amini / Sagida Bibi / Sandra Adele / Sile Ann Johnson / Bede Constantinides / Hermione Webster / Nigel Temperton / Paul Klenerman / Eleanor Barnes / Susanna J Dunachie / Derrick Crook / Andrew J Pollard / Teresa Lambe / Philip Goulder / / Neil G Paterson / Mark A Williams / David R Hall / Juthathip Mongkolsapaya / Elizabeth E Fry / Wanwisa Dejnirattisai / Jingshan Ren / David I Stuart / Gavin R Screaton /   Abstract: Highly transmissible Omicron variants of SARS-CoV-2 currently dominate globally. Here, we compare neutralization of Omicron BA.1, BA.1.1, and BA.2. BA.2 RBD has slightly higher ACE2 affinity than BA. ...Highly transmissible Omicron variants of SARS-CoV-2 currently dominate globally. Here, we compare neutralization of Omicron BA.1, BA.1.1, and BA.2. BA.2 RBD has slightly higher ACE2 affinity than BA.1 and slightly reduced neutralization by vaccine serum, possibly associated with its increased transmissibility. Neutralization differences between sub-lineages for mAbs (including therapeutics) mostly arise from variation in residues bordering the ACE2 binding site; however, more distant mutations S371F (BA.2) and R346K (BA.1.1) markedly reduce neutralization by therapeutic antibody Vir-S309. In-depth structure-and-function analyses of 27 potent RBD-binding mAbs isolated from vaccinated volunteers following breakthrough Omicron-BA.1 infection reveals that they are focused in two main clusters within the RBD, with potent right-shoulder antibodies showing increased prevalence. Selection and somatic maturation have optimized antibody potency in less-mutated epitopes and recovered potency in highly mutated epitopes. All 27 mAbs potently neutralize early pandemic strains, and many show broad reactivity with variants of concern. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14885.map.gz emd_14885.map.gz | 43.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14885-v30.xml emd-14885-v30.xml emd-14885.xml emd-14885.xml | 22 KB 22 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14885_fsc.xml emd_14885_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_14885.png emd_14885.png | 41.4 KB | ||
| Filedesc metadata |  emd-14885.cif.gz emd-14885.cif.gz | 7.4 KB | ||
| Others |  emd_14885_half_map_1.map.gz emd_14885_half_map_1.map.gz emd_14885_half_map_2.map.gz emd_14885_half_map_2.map.gz | 78.6 MB 78.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14885 http://ftp.pdbj.org/pub/emdb/structures/EMD-14885 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14885 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14885 | HTTPS FTP |
-Related structure data
| Related structure data |  7zr7MC  7zf3C  7zf4C  7zf5C  7zf6C  7zf7C  7zf8C  7zf9C  7zfaC  7zfbC  7zfcC  7zfdC  7zfeC  7zffC  7zr8C  7zr9C  7zrcC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14885.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14885.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.66 Å | ||||||||||||||||||||||||||||||||||||
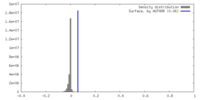
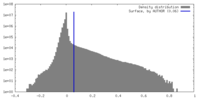
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_14885_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
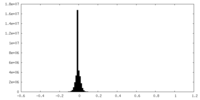
| Density Histograms |
-Half map: #1
| File | emd_14885_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
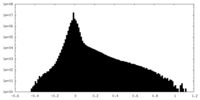
| Density Histograms |
- Sample components
Sample components
-Entire : OMI-42 FAB IN COMPLEX WITH SARS-COV-2 BETA SPIKE GLYCOPROTEIN
| Entire | Name: OMI-42 FAB IN COMPLEX WITH SARS-COV-2 BETA SPIKE GLYCOPROTEIN |
|---|---|
| Components |
|
-Supramolecule #1: OMI-42 FAB IN COMPLEX WITH SARS-COV-2 BETA SPIKE GLYCOPROTEIN
| Supramolecule | Name: OMI-42 FAB IN COMPLEX WITH SARS-COV-2 BETA SPIKE GLYCOPROTEIN type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: SARS-COV-2 BETA SPIKE GLYCOPROTEIN
| Supramolecule | Name: SARS-COV-2 BETA SPIKE GLYCOPROTEIN / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 |
-Supramolecule #3: OMI-42 FAB
| Supramolecule | Name: OMI-42 FAB / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Spike glycoprotein,Fibritin
| Macromolecule | Name: Spike glycoprotein,Fibritin / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Tequatrovirus T4 Tequatrovirus T4 |
| Molecular weight | Theoretical: 141.993984 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFVFLVLLPL VSSQCVNFTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFA NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF ...String: MFVFLVLLPL VSSQCVNFTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFA NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF EYVSQPFLMD LEGKQGNFKN LREFVFKNID GYFKIYSKHT PINLVRGLPQ GFSALEPLVD LPIGINITRF QT LHISYLT PGDSSSGWTA GAAAYYVGYL QPRTFLLKYN ENGTITDAVD CALDPLSETK CTLKSFTVEK GIYQTSNFRV QPT ESIVRF PNITNLCPFG EVFNATRFAS VYAWNRKRIS NCVADYSVLY NSASFSTFKC YGVSPTKLND LCFTNVYADS FVIR GDEVR QIAPGQTGNI ADYNYKLPDD FTGCVIAWNS NNLDSKVGGN YNYLYRLFRK SNLKPFERDI STEIYQAGST PCNGV KGFN CYFPLQSYGF QPTYGVGYQP YRVVVLSFEL LHAPATVCGP KKSTNLVKNK CVNFNFNGLT GTGVLTESNK KFLPFQ QFG RDIADTTDAV RDPQTLEILD ITPCSFGGVS VITPGTNTSN QVAVLYQGVN CTEVPVAIHA DQLTPTWRVY STGSNVF QT RAGCLIGAEH VNNSYECDIP IGAGICASYQ TQTNSPGSAS SVASQSIIAY TMSLGVENSV AYSNNSIAIP TNFTISVT T EILPVSMTKT SVDCTMYICG DSTECSNLLL QYGSFCTQLN RALTGIAVEQ DKNTQEVFAQ VKQIYKTPPI KDFGGFNFS QILPDPSKPS KRSFIEDLLF NKVTLADAGF IKQYGDCLGD IAARDLICAQ KFNGLTVLPP LLTDEMIAQY TSALLAGTIT SGWTFGAGA ALQIPFAMQM AYRFNGIGVT QNVLYENQKL IANQFNSAIG KIQDSLSSTA SALGKLQDVV NQNAQALNTL V KQLSSNFG AISSVLNDIL SRLDPPEAEV QIDRLITGRL QSLQTYVTQQ LIRAAEIRAS ANLAATKMSE CVLGQSKRVD FC GKGYHLM SFPQSAPHGV VFLHVTYVPA QEKNFTTAPA ICHDGKAHFP REGVFVSNGT HWFVTQRNFY EPQIITTDNT FVS GNCDVV IGIVNNTVYD PLQPELDSFK EELDKYFKNH TSPDVDLGDI SGINASVVNI QKEIDRLNEV AKNLNESLID LQEL GKYEQ GSGYIPEAPR DGQAYVRKDG EWVLLSTFLG RSLEVLFQGP GHHHHHHHHG SAWSHPQFEK GGGSGGGSGG SAWSH PQFE K UniProtKB:  Spike glycoprotein, Fibritin Spike glycoprotein, Fibritin |
-Macromolecule #2: Omi-42 heavy chain
| Macromolecule | Name: Omi-42 heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.541031 KDa |
| Recombinant expression | Organism:   Cricetulus griseus (Chinese hamster) Cricetulus griseus (Chinese hamster) |
| Sequence | String: EVQLLETGGG LVQPGRSLRL SCAASGFPFD DYAIHWVRLA PGKGLEWVSS ISWDSGSIGY ADSVKGRFTI SRDNAKNSLY LQMNSLRAE DTALYYCAKG AFPGYSSGWY YGLDVWGQGA TVTVSS |
-Macromolecule #3: Omi-42 light chain
| Macromolecule | Name: Omi-42 light chain / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.286393 KDa |
| Recombinant expression | Organism:   Cricetulus griseus (Chinese hamster) Cricetulus griseus (Chinese hamster) |
| Sequence | String: QSVVTQPPSA SGSLGQSVTI SCTGTSSDVG GYNYVSWYQQ HPGKAPKLMI FEVSKRPSGV PDRFSGSKSG NTASLTVSGL QAEDEADYY CSSYAGNKGV FGGGTKLTVL |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 30 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: C-flat-2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Detector mode: INTEGRATING / Number grids imaged: 1 / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)