[English] 日本語
 Yorodumi
Yorodumi- EMDB-14504: Three-dimensional structure of myosin binding protein C in rat ca... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Three-dimensional structure of myosin binding protein C in rat cardiac muscle | |||||||||
 Map data Map data | Subtomogram average of a 430 Angstrom repeat of C-zone | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | muscle regulation / C-protiein / MyBP-C /  hypertrophic cardiomyopathy / hypertrophic cardiomyopathy /  STRUCTURAL PROTEIN STRUCTURAL PROTEIN | |||||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | |||||||||
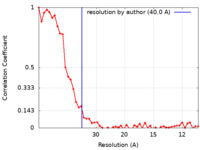
| Method | subtomogram averaging /  cryo EM / Resolution: 40.0 Å cryo EM / Resolution: 40.0 Å | |||||||||
 Authors Authors | Luther PK / Morris EP / Huang X / Jun L | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: J Muscle Res Cell Motil / Year: 2023 Journal: J Muscle Res Cell Motil / Year: 2023Title: Cryo-electron tomography of intact cardiac muscle reveals myosin binding protein-C linking myosin and actin filaments. Authors: Xinrui Huang / Iratxe Torre / Michele Chiappi / Zhan Yin / Anupama Vydyanath / Shuangyi Cao / Oliver Raschdorf / Morgan Beeby / Bonnie Quigley / Pieter P de Tombe / Jun Liu / Edward P Morris ...Authors: Xinrui Huang / Iratxe Torre / Michele Chiappi / Zhan Yin / Anupama Vydyanath / Shuangyi Cao / Oliver Raschdorf / Morgan Beeby / Bonnie Quigley / Pieter P de Tombe / Jun Liu / Edward P Morris / Pradeep K Luther /      Abstract: Myosin binding protein C (MyBP-C) is an accessory protein of the thick filament in vertebrate cardiac muscle arranged over 9 stripes of intervals of 430 Å in each half of the A-band in the region ...Myosin binding protein C (MyBP-C) is an accessory protein of the thick filament in vertebrate cardiac muscle arranged over 9 stripes of intervals of 430 Å in each half of the A-band in the region called the C-zone. Mutations in cardiac MyBP-C are a leading cause of hypertrophic cardiomyopathy the mechanism of which is unknown. It is a rod-shaped protein composed of 10 or 11 immunoglobulin- or fibronectin-like domains labelled C0 to C10 which binds to the thick filament via its C-terminal region. MyBP-C regulates contraction in a phosphorylation dependent fashion that may be through binding of its N-terminal domains with myosin or actin. Understanding the 3D organisation of MyBP-C in the sarcomere environment may provide new light on its function. We report here the fine structure of MyBP-C in relaxed rat cardiac muscle by cryo-electron tomography and subtomogram averaging of refrozen Tokuyasu cryosections. We find that on average MyBP-C connects via its distal end to actin across a disc perpendicular to the thick filament. The path of MyBP-C suggests that the central domains may interact with myosin heads. Surprisingly MyBP-C at Stripe 4 is different; it has weaker density than the other stripes which could result from a mainly axial or wavy path. Given that the same feature at Stripe 4 can also be found in several mammalian cardiac muscles and in some skeletal muscles, our finding may have broader implication and significance. In the D-zone, we show the first demonstration of myosin crowns arranged on a uniform 143 Å repeat. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14504.map.gz emd_14504.map.gz | 6.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14504-v30.xml emd-14504-v30.xml emd-14504.xml emd-14504.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14504_fsc.xml emd_14504_fsc.xml | 5.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_14504.png emd_14504.png | 75.3 KB | ||
| Filedesc metadata |  emd-14504.cif.gz emd-14504.cif.gz | 4.9 KB | ||
| Others |  emd_14504_half_map_1.map.gz emd_14504_half_map_1.map.gz emd_14504_half_map_2.map.gz emd_14504_half_map_2.map.gz | 2.6 MB 2.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14504 http://ftp.pdbj.org/pub/emdb/structures/EMD-14504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14504 | HTTPS FTP |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_14504.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14504.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Subtomogram average of a 430 Angstrom repeat of C-zone | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 5.14 Å | ||||||||||||||||||||||||||||||||||||
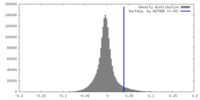
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_14504_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
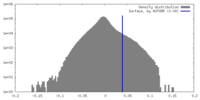
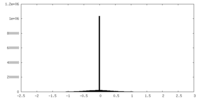
| Density Histograms |
-Half map: #2
| File | emd_14504_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
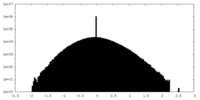
| Density Histograms |
- Sample components
Sample components
-Entire : Tokuyasu cryosection of cardiac muscle
| Entire | Name: Tokuyasu cryosection of cardiac muscle |
|---|---|
| Components |
|
-Supramolecule #1: Tokuyasu cryosection of cardiac muscle
| Supramolecule | Name: Tokuyasu cryosection of cardiac muscle / type: tissue / ID: 1 / Parent: 0 / Details: Longitudinal section of cardiac muscle |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) / Strain: Sprague Dawley / Organ: Heart / Tissue: Trabecula Rattus norvegicus (Norway rat) / Strain: Sprague Dawley / Organ: Heart / Tissue: Trabecula |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | tissue |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
Details: Krebs buffer was made fresh from concentrated components. It was aerated for 1/2 hour. | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Homemade / Material: NICKEL / Mesh: 200 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: CONTINUOUS / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: FORMVAR / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.03 kPa Details: The grid was coated with gold particles prior to freezing (recipe of Slot and Geuze). | ||||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||||||||||||||||||||
| Details | Trabeculae and papillary muscles were dissected from a rat heart |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 26000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 5.5 µm / Nominal defocus min: 4.0 µm / Nominal magnification: 26000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 5.5 µm / Nominal defocus min: 4.0 µm / Nominal magnification: 26000 |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Temperature | Min: 88.0 K / Max: 88.0 K |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3840 pixel / Digitization - Dimensions - Height: 3712 pixel / Digitization - Frames/image: 1-12 / Number grids imaged: 1 / Average exposure time: 12.0 sec. / Average electron dose: 2.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: OTHER |
|---|
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)