+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human monomeric IgM-Fc | |||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
| Function / homology | Isoform 2 of Immunoglobulin heavy constant mu Function and homology information Function and homology information | |||||||||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | |||||||||||||||||||||
 Authors Authors | Chen Q / Rosenthal P / Tolar P | |||||||||||||||||||||
| Funding support |  United Kingdom, 6 items United Kingdom, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Cryomicroscopy reveals the structural basis for a flexible hinge motion in the immunoglobulin M pentamer. Authors: Qu Chen / Rajesh Menon / Lesley J Calder / Pavel Tolar / Peter B Rosenthal /  Abstract: Immunoglobulin M (IgM) is the most ancient of the five isotypes of immunoglobulin (Ig) molecules and serves as the first line of defence against pathogens. Here, we use cryo-EM to image the structure ...Immunoglobulin M (IgM) is the most ancient of the five isotypes of immunoglobulin (Ig) molecules and serves as the first line of defence against pathogens. Here, we use cryo-EM to image the structure of the human full-length IgM pentamer, revealing antigen binding domains flexibly attached to the asymmetric and rigid core formed by the Cμ4 and Cμ3 constant regions and the J-chain. A hinge is located at the Cμ3/Cμ2 domain interface, allowing Fabs and Cμ2 to pivot as a unit both in-plane and out-of-plane. This motion is different from that observed in IgG and IgA, where the two Fab arms are able to swing independently. A biased orientation of one pair of Fab arms results from asymmetry in the constant domain (Cμ3) at the IgM subunit interacting most extensively with the J-chain. This may influence the multi-valent binding to surface-associated antigens and complement pathway activation. By comparison, the structure of the Fc fragment in the IgM monomer is similar to that of the pentamer, but is more dynamic in the Cμ4 domain. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13922.map.gz emd_13922.map.gz | 4.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13922-v30.xml emd-13922-v30.xml emd-13922.xml emd-13922.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
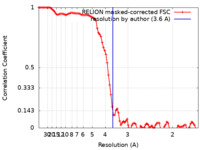
| FSC (resolution estimation) |  emd_13922_fsc.xml emd_13922_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_13922.png emd_13922.png | 55.9 KB | ||
| Masks |  emd_13922_msk_1.map emd_13922_msk_1.map | 64 MB |  Mask map Mask map | |
| Others |  emd_13922_half_map_1.map.gz emd_13922_half_map_1.map.gz emd_13922_half_map_2.map.gz emd_13922_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13922 http://ftp.pdbj.org/pub/emdb/structures/EMD-13922 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13922 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13922 | HTTPS FTP |
-Related structure data
| Related structure data |  7qdoMC  8adyC  8adzC  8ae0C  8ae2C  8ae3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13922.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13922.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.839 Å | ||||||||||||||||||||||||||||||||||||
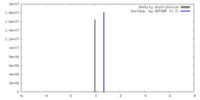
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_13922_msk_1.map emd_13922_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
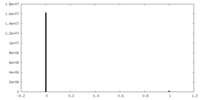
| Density Histograms |
-Half map: #2
| File | emd_13922_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
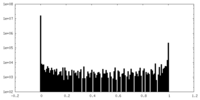
| Density Histograms |
-Half map: #1
| File | emd_13922_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
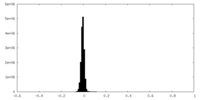
| Density Histograms |
- Sample components
Sample components
-Entire : human monomeric IgM-Fc
| Entire | Name: human monomeric IgM-Fc |
|---|---|
| Components |
|
-Supramolecule #1: human monomeric IgM-Fc
| Supramolecule | Name: human monomeric IgM-Fc / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Isoform 2 of Immunoglobulin heavy constant mu
| Macromolecule | Name: Isoform 2 of Immunoglobulin heavy constant mu / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 47.687059 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGILPSPGMP ALLSLVSLLS VLLMGCVAET GVIAELPPKV SVFVPPRDGF FGNPRKSKLI CQATGFSPRQ IQVSWLREGK QVGSGVTTD QVQAEAKESG PTTYKVTSTL TIKESDWLSQ SMFTCRVDHR GLTFQQNASS MCVPDQDTAI RVFAIPPSFA S IFLTKSTK ...String: MGILPSPGMP ALLSLVSLLS VLLMGCVAET GVIAELPPKV SVFVPPRDGF FGNPRKSKLI CQATGFSPRQ IQVSWLREGK QVGSGVTTD QVQAEAKESG PTTYKVTSTL TIKESDWLSQ SMFTCRVDHR GLTFQQNASS MCVPDQDTAI RVFAIPPSFA S IFLTKSTK LTCLVTDLTT YDSVTISWTR QNGEAVKTHT NISESHPNAT FSAVGEASIS EDDWNSGERF TCTVTHTDLP SP LKQTISR PKGVALHRPD VYLLPPAREQ LNLRESATIT CLVTGFSPAD VFVQWMQRGQ PLSPEKYVTS APMPEPQAPG RYF AHSILT VSEEEWNTGE TYTCVVAHEA LPNRVTERTV DKSTEGEVSA DEEGFENEIA QLEYEISQLE QEIQALESGG GSGG GSENL YFQGGGSWSH PQFEKGGGSG GGSGGSAWSH PQFEK |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 4.0 µm / Calibrated defocus min: 1.0 µm / Calibrated magnification: 59595 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.6 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 165000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 3.6 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average electron dose: 66.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-7qdo: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)