+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | RNA origami 5-helix tile | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  RNA / RNA /  origami / origami /  nanostructure nanostructure | |||||||||
| Biological species | synthetic construct (others) | |||||||||
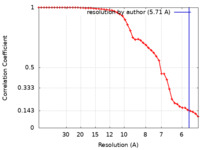
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 5.71 Å cryo EM / Resolution: 5.71 Å | |||||||||
 Authors Authors | McRae EKS / Bogglid A / Boesen T / Andersen ES | |||||||||
| Funding support |  Denmark, 1 items Denmark, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Nanotechnol / Year: 2023 Journal: Nat Nanotechnol / Year: 2023Title: Structure, folding and flexibility of co-transcriptional RNA origami. Authors: Ewan K S McRae / Helena Østergaard Rasmussen / Jianfang Liu / Andreas Bøggild / Michael T A Nguyen / Nestor Sampedro Vallina / Thomas Boesen / Jan Skov Pedersen / Gang Ren / Cody Geary / ...Authors: Ewan K S McRae / Helena Østergaard Rasmussen / Jianfang Liu / Andreas Bøggild / Michael T A Nguyen / Nestor Sampedro Vallina / Thomas Boesen / Jan Skov Pedersen / Gang Ren / Cody Geary / Ebbe Sloth Andersen /   Abstract: RNA origami is a method for designing RNA nanostructures that can self-assemble through co-transcriptional folding with applications in nanomedicine and synthetic biology. However, to advance the ...RNA origami is a method for designing RNA nanostructures that can self-assemble through co-transcriptional folding with applications in nanomedicine and synthetic biology. However, to advance the method further, an improved understanding of RNA structural properties and folding principles is required. Here we use cryogenic electron microscopy to study RNA origami sheets and bundles at sub-nanometre resolution revealing structural parameters of kissing-loop and crossover motifs, which are used to improve designs. In RNA bundle designs, we discover a kinetic folding trap that forms during folding and is only released after 10 h. Exploration of the conformational landscape of several RNA designs reveal the flexibility of helices and structural motifs. Finally, sheets and bundles are combined to construct a multidomain satellite shape, which is characterized by individual-particle cryo-electron tomography to reveal the domain flexibility. Together, the study provides a structural basis for future improvements to the design cycle of genetically encoded RNA nanodevices. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13636.map.gz emd_13636.map.gz | 4.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13636-v30.xml emd-13636-v30.xml emd-13636.xml emd-13636.xml | 20.7 KB 20.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13636_fsc.xml emd_13636_fsc.xml | 4.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_13636.png emd_13636.png | 46.8 KB | ||
| Masks |  emd_13636_msk_1.map emd_13636_msk_1.map | 8 MB |  Mask map Mask map | |
| Others |  emd_13636_additional_1.map.gz emd_13636_additional_1.map.gz emd_13636_half_map_1.map.gz emd_13636_half_map_1.map.gz emd_13636_half_map_2.map.gz emd_13636_half_map_2.map.gz | 7.6 MB 7.4 MB 7.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13636 http://ftp.pdbj.org/pub/emdb/structures/EMD-13636 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13636 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13636 | HTTPS FTP |
-Related structure data
| Related structure data |  7ptsMC  7ptkC  7ptlC  7ptqC  7qduC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13636.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13636.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.59 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_13636_msk_1.map emd_13636_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map from cryoSPARC with bfactor 306.
| File | emd_13636_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map from cryoSPARC with bfactor 306. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_13636_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_13636_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : 5 helix tile B
| Entire | Name: 5 helix tile B |
|---|---|
| Components |
|
-Supramolecule #1: 5 helix tile B
| Supramolecule | Name: 5 helix tile B / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: In vitro transcribed RNA purified by SEC. |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 180 KDa |
-Macromolecule #1: 5HT-B
| Macromolecule | Name: 5HT-B / type: rna / ID: 1 / Details: In vitro transcribed RNA / Number of copies: 1 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 179.904219 KDa |
| Sequence | String: GGAACUUAUU CAUUAUACCC CUCAUUUUCG AGUGAGGCGG AUUAGAUUCG UCUGAUCGUA GUGGUGGUGG CUUGCUUCGG CGAGCCCGG CAGGCCUUCG GGCUUGCUCA GGGGUUCGCU CCUGAGAUUA UCAUCCAACC UCCAAGGAUG GUAAUCCGGC G UCGAUUGU ...String: GGAACUUAUU CAUUAUACCC CUCAUUUUCG AGUGAGGCGG AUUAGAUUCG UCUGAUCGUA GUGGUGGUGG CUUGCUUCGG CGAGCCCGG CAGGCCUUCG GGCUUGCUCA GGGGUUCGCU CCUGAGAUUA UCAUCCAACC UCCAAGGAUG GUAAUCCGGC G UCGAUUGU AAGGUCUAAA CAAUUGACGC ACCACUACUA CAGUCCCAAC GACUGAGGGA CUCGUCGACU CGGUUAAGAU CG GAAACCG GGUCGAGGUA UAGUGAAUGA GUUCCAAUUC UCCAUUUACG GCAUCUUGUG CGAACCGAUC ACGCACGAGA UGG UCCUUA CGGGGUGUCC CAACAGUCGA GGGGCACGCA GGAUGCCUAG AAUAGACCAC UAGGUAUCCU CAGUGCGCCA UGCA AUGGA GGAGCAUGGU GCACUGCAGG GCUCCUGAAA AGGAGUCCUG GCAGAAGGGU UCGCCUUUCU CCCGUAGGGA CGACU UUCU UUGAAAAAAG GAAGUCCCAG UUUGCUUCGG CGAACUCCGA CUCUAGGAAA CUAGGGUCGG GUAGAUGGAG AAUU |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: Freshly prepared and filtered through 0.22 micron filter prior to use. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 99 % / Chamber temperature: 294 K / Instrument: LEICA EM GP Details: 3 microlitre droplet, 4 second delay before blotting, 6 second blot, 0 second delay before plunging.. | ||||||||||||
| Details | Sample was purified by size exclusion chromatography and concentrated in an Amicon spin concentrator. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 130000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 130000 |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 1331 / Average exposure time: 1.5 sec. / Average electron dose: 60.0 e/Å2 / Details: Images were collected as 56 frame movies. |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Details | Helical templates and tetraloops were generated in RNAbuild. Kissing loops were modelling based on the PDB:2D1B structure. A combination of PHENIX RSR and ISOLDE were used to arrive at the final structure. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 306 / Target criteria: Correlation Coefficient |
| Output model |  PDB-7pts: |
 Movie
Movie Controller
Controller













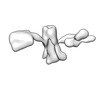
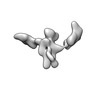
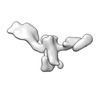

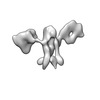
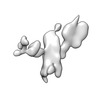
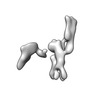






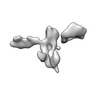
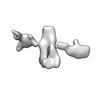
 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)