[English] 日本語
 Yorodumi
Yorodumi- EMDB-12874: CryoEM structure of the outer membrane secretin pore pIV from the... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12874 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the outer membrane secretin pore pIV from the f1 filamentous bacteriophage. | |||||||||
 Map data Map data | Postprocessed masked map from Relion | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationviral extrusion /  protein secretion / host cell membrane / membrane => GO:0016020 protein secretion / host cell membrane / membrane => GO:0016020Similarity search - Function | |||||||||
| Biological species |  Inoviridae sp. (virus) / Inoviridae sp. (virus) /  Enterobacteria phage f1 (virus) Enterobacteria phage f1 (virus) | |||||||||
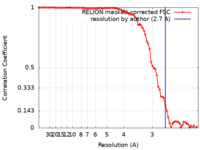
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.7 Å cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Conners R / Gold VAM | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: CryoEM structure of the outer membrane secretin channel pIV from the f1 filamentous bacteriophage. Authors: Rebecca Conners / Mathew McLaren / Urszula Łapińska / Kelly Sanders / M Rhia L Stone / Mark A T Blaskovich / Stefano Pagliara / Bertram Daum / Jasna Rakonjac / Vicki A M Gold /    Abstract: The Ff family of filamentous bacteriophages infect gram-negative bacteria, but do not cause lysis of their host cell. Instead, new virions are extruded via the phage-encoded pIV protein, which has ...The Ff family of filamentous bacteriophages infect gram-negative bacteria, but do not cause lysis of their host cell. Instead, new virions are extruded via the phage-encoded pIV protein, which has homology with bacterial secretins. Here, we determine the structure of pIV from the f1 filamentous bacteriophage at 2.7 Å resolution by cryo-electron microscopy, the first near-atomic structure of a phage secretin. Fifteen f1 pIV subunits assemble to form a gated channel in the bacterial outer membrane, with associated soluble domains projecting into the periplasm. We model channel opening and propose a mechanism for phage egress. By single-cell microfluidics experiments, we demonstrate the potential for secretins such as pIV to be used as adjuvants to increase the uptake and efficacy of antibiotics in bacteria. Finally, we compare the f1 pIV structure to its homologues to reveal similarities and differences between phage and bacterial secretins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12874.map.gz emd_12874.map.gz | 7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12874-v30.xml emd-12874-v30.xml emd-12874.xml emd-12874.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12874_fsc.xml emd_12874_fsc.xml | 8 KB | Display |  FSC data file FSC data file |
| Images |  emd_12874.png emd_12874.png | 302.8 KB | ||
| Others |  emd_12874_additional_1.map.gz emd_12874_additional_1.map.gz emd_12874_additional_2.map.gz emd_12874_additional_2.map.gz emd_12874_additional_3.map.gz emd_12874_additional_3.map.gz | 36.2 MB 32.9 MB 32.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12874 http://ftp.pdbj.org/pub/emdb/structures/EMD-12874 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12874 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12874 | HTTPS FTP |
-Related structure data
| Related structure data |  7ofhMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10807 (Title: CryoEM structure of the outer membrane secretin channel pIV from the f1 filamentous bacteriophage EMPIAR-10807 (Title: CryoEM structure of the outer membrane secretin channel pIV from the f1 filamentous bacteriophageData size: 7.0 TB Data #1: Micrographs of the secretin protein, pIV, from the f1 filamentous bacteriophage (dataset1) [micrographs - multiframe] Data #2: Micrographs of the secretin protein, pIV, from the f1 filamentous bacteriophage (dataset2) [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12874.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12874.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed masked map from Relion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.072 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Postprocessed map after DeepEMhancer
| File | emd_12874_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map after DeepEMhancer | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half map1
| File | emd_12874_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half map2
| File | emd_12874_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Virion export protein pIV
| Entire | Name: Virion export protein pIV |
|---|---|
| Components |
|
-Supramolecule #1: Virion export protein pIV
| Supramolecule | Name: Virion export protein pIV / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: From Inoviridae sp. f1 |
|---|---|
| Source (natural) | Organism:  Inoviridae sp. (virus) Inoviridae sp. (virus) |
| Molecular weight | Theoretical: 669.7 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) / Recombinant strain: TG1 / Recombinant plasmid: pPMR132 Escherichia coli (E. coli) / Recombinant strain: TG1 / Recombinant plasmid: pPMR132 |
-Macromolecule #1: Virion export protein
| Macromolecule | Name: Virion export protein / type: protein_or_peptide / ID: 1 Details: S9P, D49N, I66N are naturally occurring polymorphisms. Number of copies: 15 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Enterobacteria phage f1 (virus) Enterobacteria phage f1 (virus) |
| Molecular weight | Theoretical: 44.657707 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: QVIEMNNSPL RDFVTWYSKQ TGESVIVSPD VKGTVTVYSS DVKPENLRNF FISVLRANNF DMVGSNPSII QKYNPNNQDY IDELPSSDN QEYDDNSAPS GGFFVPQNDN VTQTFKINNV RAKDLIRVVE LFVKSNTSKS SNVLSVDGSN LLVVSAPKDI L DNLPQFLS ...String: QVIEMNNSPL RDFVTWYSKQ TGESVIVSPD VKGTVTVYSS DVKPENLRNF FISVLRANNF DMVGSNPSII QKYNPNNQDY IDELPSSDN QEYDDNSAPS GGFFVPQNDN VTQTFKINNV RAKDLIRVVE LFVKSNTSKS SNVLSVDGSN LLVVSAPKDI L DNLPQFLS TVDLPTDQIL IEGLIFEVQQ GDALDFSFAA GSQRGTVAGG VNTDRLTSVL SSAGGSFGIF NGDVLGLSVR AL KTNSHSK ILSVPRILTL SGQKGSISVG QNVPFITGRV TGESANVNNP FQTVERQNVG ISMSVFPVAM ASAHHHHHHH GGN IVLDIT IKADSLSSST QASDVITNQR SIATTVNLRD GQTLLLGGLT DYKNTSQDSG VPFLSKIPLI GLLFSSRSDS NEES TLYVL VKATIVRAL |
-Macromolecule #2: 3-[(3-CHOLAMIDOPROPYL)DIMETHYLAMMONIO]-1-PROPANESULFONATE
| Macromolecule | Name: 3-[(3-CHOLAMIDOPROPYL)DIMETHYLAMMONIO]-1-PROPANESULFONATE type: ligand / ID: 2 / Number of copies: 30 / Formula: CPS |
|---|---|
| Molecular weight | Theoretical: 614.877 Da |
| Chemical component information |  ChemComp-CPS: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: PELCO Ultrathin Carbon with Lacey Carbon / Mesh: 300 / Support film - Material: GRAPHENE OXIDE / Support film - topology: LACEY | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot force 0, blot time 4sec. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 81000 Bright-field microscopy / Nominal magnification: 81000 |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average exposure time: 3.52 sec. / Average electron dose: 42.059 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z
Z Y
Y X
X