[English] 日本語
 Yorodumi
Yorodumi- EMDB-12368: CryoEM structure of the human Separase-Cdk1-cyclin B1-Cks1 complex -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12368 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the human Separase-Cdk1-cyclin B1-Cks1 complex | |||||||||
 Map data Map data | Separase-Cdk1-cyclin B1-Cks1 complex (postprocessed). | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of mitotic sister chromatid separation / negative regulation of sister chromatid cohesion /  separase / regulation of Schwann cell differentiation / pronuclear fusion / cyclin B1-CDK1 complex / positive regulation of mitochondrial ATP synthesis coupled electron transport / Mitotic Prophase / positive regulation of mitotic sister chromatid segregation / meiotic chromosome separation ...negative regulation of mitotic sister chromatid separation / negative regulation of sister chromatid cohesion / separase / regulation of Schwann cell differentiation / pronuclear fusion / cyclin B1-CDK1 complex / positive regulation of mitochondrial ATP synthesis coupled electron transport / Mitotic Prophase / positive regulation of mitotic sister chromatid segregation / meiotic chromosome separation ...negative regulation of mitotic sister chromatid separation / negative regulation of sister chromatid cohesion /  separase / regulation of Schwann cell differentiation / pronuclear fusion / cyclin B1-CDK1 complex / positive regulation of mitochondrial ATP synthesis coupled electron transport / Mitotic Prophase / positive regulation of mitotic sister chromatid segregation / meiotic chromosome separation / histone kinase activity / Golgi disassembly / microtubule cytoskeleton organization involved in mitosis / G2/M DNA replication checkpoint / E2F-enabled inhibition of pre-replication complex formation / ventricular cardiac muscle cell development / Depolymerization of the Nuclear Lamina / positive regulation of attachment of spindle microtubules to kinetochore / MASTL Facilitates Mitotic Progression / regulation of mitotic cell cycle spindle assembly checkpoint / establishment of mitotic spindle localization / Activation of NIMA Kinases NEK9, NEK6, NEK7 / homologous chromosome segregation / Phosphorylation of Emi1 / Phosphorylation of proteins involved in the G2/M transition by Cyclin A:Cdc2 complexes / separase / regulation of Schwann cell differentiation / pronuclear fusion / cyclin B1-CDK1 complex / positive regulation of mitochondrial ATP synthesis coupled electron transport / Mitotic Prophase / positive regulation of mitotic sister chromatid segregation / meiotic chromosome separation / histone kinase activity / Golgi disassembly / microtubule cytoskeleton organization involved in mitosis / G2/M DNA replication checkpoint / E2F-enabled inhibition of pre-replication complex formation / ventricular cardiac muscle cell development / Depolymerization of the Nuclear Lamina / positive regulation of attachment of spindle microtubules to kinetochore / MASTL Facilitates Mitotic Progression / regulation of mitotic cell cycle spindle assembly checkpoint / establishment of mitotic spindle localization / Activation of NIMA Kinases NEK9, NEK6, NEK7 / homologous chromosome segregation / Phosphorylation of Emi1 / Phosphorylation of proteins involved in the G2/M transition by Cyclin A:Cdc2 complexes /  patched binding / cyclin A2-CDK1 complex / meiotic spindle organization / positive regulation of mitotic metaphase/anaphase transition / Nuclear Pore Complex (NPC) Disassembly / Transcriptional regulation by RUNX2 / outer kinetochore / Phosphorylation of the APC/C / mitotic cell cycle phase transition / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / Initiation of Nuclear Envelope (NE) Reformation / protein localization to kinetochore / Polo-like kinase mediated events / Golgi Cisternae Pericentriolar Stack Reorganization / cyclin-dependent protein serine/threonine kinase activator activity / chromosome condensation / Condensation of Prometaphase Chromosomes / response to copper ion / patched binding / cyclin A2-CDK1 complex / meiotic spindle organization / positive regulation of mitotic metaphase/anaphase transition / Nuclear Pore Complex (NPC) Disassembly / Transcriptional regulation by RUNX2 / outer kinetochore / Phosphorylation of the APC/C / mitotic cell cycle phase transition / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / Initiation of Nuclear Envelope (NE) Reformation / protein localization to kinetochore / Polo-like kinase mediated events / Golgi Cisternae Pericentriolar Stack Reorganization / cyclin-dependent protein serine/threonine kinase activator activity / chromosome condensation / Condensation of Prometaphase Chromosomes / response to copper ion /  centrosome cycle / [RNA-polymerase]-subunit kinase / cyclin-dependent protein serine/threonine kinase regulator activity / centrosome cycle / [RNA-polymerase]-subunit kinase / cyclin-dependent protein serine/threonine kinase regulator activity /  SCF ubiquitin ligase complex / mitotic metaphase chromosome alignment / cysteine-type endopeptidase inhibitor activity / G1/S-Specific Transcription / cyclin-dependent protein kinase activity / MAPK3 (ERK1) activation / response to amine / ubiquitin-like protein ligase binding / mitotic sister chromatid segregation / mitotic G2 DNA damage checkpoint signaling / SCF ubiquitin ligase complex / mitotic metaphase chromosome alignment / cysteine-type endopeptidase inhibitor activity / G1/S-Specific Transcription / cyclin-dependent protein kinase activity / MAPK3 (ERK1) activation / response to amine / ubiquitin-like protein ligase binding / mitotic sister chromatid segregation / mitotic G2 DNA damage checkpoint signaling /  regulation of embryonic development / Regulation of APC/C activators between G1/S and early anaphase / mitotic cytokinesis / cellular response to organic cyclic compound / chromosome organization / cyclin-dependent protein kinase holoenzyme complex / response to axon injury / regulation of embryonic development / Regulation of APC/C activators between G1/S and early anaphase / mitotic cytokinesis / cellular response to organic cyclic compound / chromosome organization / cyclin-dependent protein kinase holoenzyme complex / response to axon injury /  cyclin-dependent kinase / animal organ regeneration / cyclin-dependent protein serine/threonine kinase activity / response to cadmium ion / Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex / cysteine-type peptidase activity / cyclin-dependent kinase / animal organ regeneration / cyclin-dependent protein serine/threonine kinase activity / response to cadmium ion / Chk1/Chk2(Cds1) mediated inactivation of Cyclin B:Cdk1 complex / cysteine-type peptidase activity /  catalytic activity / Cyclin A/B1/B2 associated events during G2/M transition / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / positive regulation of cardiac muscle cell proliferation / Recruitment of mitotic centrosome proteins and complexes / ERK1 and ERK2 cascade / catalytic activity / Cyclin A/B1/B2 associated events during G2/M transition / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / positive regulation of cardiac muscle cell proliferation / Recruitment of mitotic centrosome proteins and complexes / ERK1 and ERK2 cascade /  Hsp70 protein binding / Resolution of Sister Chromatid Cohesion / Recruitment of NuMA to mitotic centrosomes / epithelial cell differentiation / APC/C:Cdc20 mediated degradation of Cyclin B / Anchoring of the basal body to the plasma membrane / positive regulation of G2/M transition of mitotic cell cycle / regulation of mitotic cell cycle / Hsp70 protein binding / Resolution of Sister Chromatid Cohesion / Recruitment of NuMA to mitotic centrosomes / epithelial cell differentiation / APC/C:Cdc20 mediated degradation of Cyclin B / Anchoring of the basal body to the plasma membrane / positive regulation of G2/M transition of mitotic cell cycle / regulation of mitotic cell cycle /  cyclin binding / positive regulation of mitotic cell cycle / RNA polymerase II CTD heptapeptide repeat kinase activity / AURKA Activation by TPX2 / mitotic spindle organization / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / Condensation of Prophase Chromosomes / positive regulation of DNA replication / response to activity / cyclin binding / positive regulation of mitotic cell cycle / RNA polymerase II CTD heptapeptide repeat kinase activity / AURKA Activation by TPX2 / mitotic spindle organization / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / Condensation of Prophase Chromosomes / positive regulation of DNA replication / response to activity /  ubiquitin binding / APC/C:Cdc20 mediated degradation of Securin / molecular function activator activity / spindle microtubule / Cdc20:Phospho-APC/C mediated degradation of Cyclin A / peptidyl-threonine phosphorylation / G1/S transition of mitotic cell cycle ubiquitin binding / APC/C:Cdc20 mediated degradation of Securin / molecular function activator activity / spindle microtubule / Cdc20:Phospho-APC/C mediated degradation of Cyclin A / peptidyl-threonine phosphorylation / G1/S transition of mitotic cell cycleSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Yu J / Raia P / Ghent CM / Raisch T / Sadian Y / Barford D / Raunser S / Morgan DO / Boland A | |||||||||
| Funding support |  Switzerland, Switzerland,  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Structural basis of human separase regulation by securin and CDK1-cyclin B1. Authors: Jun Yu / Pierre Raia / Chloe M Ghent / Tobias Raisch / Yashar Sadian / Simone Cavadini / Pramod M Sabale / David Barford / Stefan Raunser / David O Morgan / Andreas Boland /     Abstract: In early mitosis, the duplicated chromosomes are held together by the ring-shaped cohesin complex. Separation of chromosomes during anaphase is triggered by separase-a large cysteine endopeptidase ...In early mitosis, the duplicated chromosomes are held together by the ring-shaped cohesin complex. Separation of chromosomes during anaphase is triggered by separase-a large cysteine endopeptidase that cleaves the cohesin subunit SCC1 (also known as RAD21). Separase is activated by degradation of its inhibitors, securin and cyclin B, but the molecular mechanisms of separase regulation are not clear. Here we used cryogenic electron microscopy to determine the structures of human separase in complex with either securin or CDK1-cyclin B1-CKS1. In both complexes, separase is inhibited by pseudosubstrate motifs that block substrate binding at the catalytic site and at nearby docking sites. As in Caenorhabditis elegans and yeast, human securin contains its own pseudosubstrate motifs. By contrast, CDK1-cyclin B1 inhibits separase by deploying pseudosubstrate motifs from intrinsically disordered loops in separase itself. One autoinhibitory loop is oriented by CDK1-cyclin B1 to block the catalytic sites of both separase and CDK1. Another autoinhibitory loop blocks substrate docking in a cleft adjacent to the separase catalytic site. A third separase loop contains a phosphoserine that promotes complex assembly by binding to a conserved phosphate-binding pocket in cyclin B1. Our study reveals the diverse array of mechanisms by which securin and CDK1-cyclin B1 bind and inhibit separase, providing the molecular basis for the robust control of chromosome segregation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12368.map.gz emd_12368.map.gz | 12.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12368-v30.xml emd-12368-v30.xml emd-12368.xml emd-12368.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_12368.png emd_12368.png | 166.1 KB | ||
| Others |  emd_12368_additional_1.map.gz emd_12368_additional_1.map.gz | 140.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12368 http://ftp.pdbj.org/pub/emdb/structures/EMD-12368 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12368 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12368 | HTTPS FTP |
-Related structure data
| Related structure data |  7nj0MC  7nj1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12368.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12368.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Separase-Cdk1-cyclin B1-Cks1 complex (postprocessed). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Separase-Cdk1-cyclin B1-Cks1 complex (unsharpened).
| File | emd_12368_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Separase-Cdk1-cyclin B1-Cks1 complex (unsharpened). | ||||||||||||
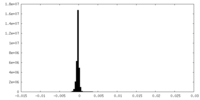
| Projections & Slices |
| ||||||||||||
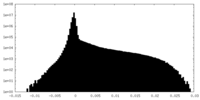
| Density Histograms |
- Sample components
Sample components
-Entire : Mutual inhibitory complex of human separase-Cdk1-cyclin B1-Cks1 (...
| Entire | Name: Mutual inhibitory complex of human separase-Cdk1-cyclin B1-Cks1 (CCC) complex. |
|---|---|
| Components |
|
-Supramolecule #1: Mutual inhibitory complex of human separase-Cdk1-cyclin B1-Cks1 (...
| Supramolecule | Name: Mutual inhibitory complex of human separase-Cdk1-cyclin B1-Cks1 (CCC) complex. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
-Macromolecule #1: Securin,Separin
| Macromolecule | Name: Securin,Separin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number:  separase separase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 244.677328 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MQLGPPSPVK MPSPPWESNL LQSPSSILST LDVELPPVCC DIDIGGSGGS GGGSGGGSGE NLYFQGGGSG GSGMRSFKRV NFGTLLSSQ KEAEELLPDL KEFLSNPPAG FPSSRSDAER RQACDAILRA CNQQLTAKLA CPRHLGSLLE LAELACDGYL V STPQRPPL ...String: MQLGPPSPVK MPSPPWESNL LQSPSSILST LDVELPPVCC DIDIGGSGGS GGGSGGGSGE NLYFQGGGSG GSGMRSFKRV NFGTLLSSQ KEAEELLPDL KEFLSNPPAG FPSSRSDAER RQACDAILRA CNQQLTAKLA CPRHLGSLLE LAELACDGYL V STPQRPPL YLERILFVLL RNAAAQGSPE VTLRLAQPLH ACLVQCSREA APQDYEAVAR GSFSLLWKGA EALLERRAAF AA RLKALSF LVLLEDESTP CEVPHFASPT ACRAVAAHQL FDASGHGLNE ADADFLDDLL SRHVIRALVG ERGSSSGLLS PQR ALCLLE LTLEHCRRFC WSRHHDKAIS AVEKAHSYLR NTNLAPSLQL CQLGVKLLQV GEEGPQAVAK LLIKASAVLS KSME APSPP LRALYESCQF FLSGLERGTK RRYRLDAILS LFAFLGGYCS LLQQLRDDGV YGGSSKQQQS FLQMYFQGLH LYTVV VYDF AQGCQIVDLA DLTQLVDSCK STVVWMLEAL EGLSGQELTD HMGMTASYTS NLAYSFYSHK LYAEACAISE PLCQHL GLV KPGTYPEVPP EKLHRCFRLQ VESLKKLGKQ AQGCKMVILW LAALQPCSPE HMAEPVTFWV RVKMDAARAG DKELQLK TL RDSLSGWDPE TLALLLREEL QAYKAVRADT GQERFNIICD LLELSPEETP AGAWARATHL VELAQVLCYH DFTQQTNC S ALDAIREALQ LLDSVRPEAQ ARDQLLDDKA QALLWLYICT LEAKIQEGIE RDRRAQAPGN LEEFEVNDLN YEDKLQEDR FLYSNIAFNL AADAAQSKCL DQALALWKEL LTKGQAPAVR CLQQTAASLQ ILAALYQLVA KPMQALEVLL LLRIVSERLK DHSKAAGSS CHITQLLLTL GCPSYAQLHL EEAASSLKHL DQTTDTYLLL SLTCDLLRSQ LYWTHQKVTK GVSLLLSVLR D PALQKSSK AWYLLRVQVL QLVAAYLSLP SNNLSHSLWE QLCAQGWQTP EIALIDSHKL LRSIILLLMG SDILSTQKAA VE TSFLDYG ENLVQKWQVL SEVLSCSEKL VCHLGRLGSV SEAKAFCLEA LKLTTKLQIP RQCALFLVLK GELELARNDI DLC QSDLQQ VLFLLESCTE FGGVTQHLDS VKKVHLQKGK QQAQVPCPPQ LPEEELFLRG PALELVATVA KEPGPIAPST NS (SEP)PVLKTK PQPIPNFLSH SPTCDCSLCA SPVLTAVCLR WVLVTAGVRL AMGHQAQGLD LLQVVLKGCP EAAERLTQA LQASLNHKTP PSLVPSLLDE ILAQAYTLLA LEGLNQPSNE SLQKVLQSGL KFVAARIPHL EPWRASLLLI WALTKLGGLS CCTTQLFAS SWGWQPPLIK SVPGSEPSKT QGQKRSGRGR QKLASAPLSL NNTSQKGLEG RGLPCTPKPP DRIRQAGPHV P FTVFEEVC PTESKPEVPQ APRVQQRVQT RLKVNFSDDS DLEDPVSAEA WLAEEPKRRG TASRGRGRAR KGLSLKTDAV VA PGSAPGN PGLNGRSRRA KKVASRHCEE RRPQRASDQA RPGPEIMRTI PEEELTDNWR KMSFEILRGS DGEDSASGGK TPA PGPEAA SGEWELLRLD SSKKKLPSPC PDKESDKDLG PRLQLPSAPV ATGLSTLDSI CDSLSVAFRG ISHCPPSGLY AHLC RFLAL CLGHRDPYAT AFLVTESVSI TCRHQLLTHL HRQLSKAQKH RGSLEIADQL QGLSLQEMPG DVPLARIQRL FSFRA LESG HFPQPEKESF QERLALIPSG VTVCVLALAT LQPGTVGNTL LLTRLEKDSP PVSVQIPTGQ NKLHLRSVLN EFDAIQ KAQ KENSSCTDKR EWWTGRLALD HRMEVLIASL EKSVLGCWKG LLLPSSEEPG PAQEASRLQE LLQDCGWKYP DRTLLKI ML SGAGALTPQD IQALAYGLCP TQPERAQELL NEAVGRLQGL TVPSNSHLVL VLDKDLQKLP WESMPSLQAL PVTRLPSF R FLLSYSIIKE YGASPVLSQG VDPRSTFYVL NPHNNLSSTE EQFRANFSSE AGWRGVVGEV PRPEQVQEAL TKHDLYIYA GHGAGARFLD GQAVLRLSCR AVALLFGSSS AALAVHGNLE GAGIVLKYIM AGCPLFLGNL WDVTDRDIDR YTEALLQGWL GAGPGAPLL YYVNQARQAP RLKYLIGAAP IAYGLPVSLR SSLAEENLYF QSWSHPQFEK GGGSGGGSGG GSWSHPQFEK |
-Macromolecule #2: Cyclin-dependent kinase 1
| Macromolecule | Name: Cyclin-dependent kinase 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number:  cyclin-dependent kinase cyclin-dependent kinase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 36.667098 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MEDYTKIEKI GEGTYGVVYK GRHKTTGQVV AMKKIRLESE EEGVPSTAIR EISLLKELRH PNIVSLQDVL MQDSRLYLIF EFLSMDLKK YLDSIPPGQY MDSSLVKSYL YQILQGIVFC HSRRVLHRDL KPQNLLIDDK GTIKLADFGL ARAFGIPIRV Y (TPO)HEVVTLW ...String: MEDYTKIEKI GEGTYGVVYK GRHKTTGQVV AMKKIRLESE EEGVPSTAIR EISLLKELRH PNIVSLQDVL MQDSRLYLIF EFLSMDLKK YLDSIPPGQY MDSSLVKSYL YQILQGIVFC HSRRVLHRDL KPQNLLIDDK GTIKLADFGL ARAFGIPIRV Y (TPO)HEVVTLW YRSPEVLLGS ARYSTPVDIW SIGTIFAELA TKKPLFHGDS EIDQLFRIFR ALGTPNNEVW PEVESLQD Y KNTFPKWKPG SLASHVKNLD ENGLDLLSKM LIYDPAKRIS GKMALNHPYF NDLDNQIKKM IAAEALEVLF QGPHHHHHH HH |
-Macromolecule #3: G2/mitotic-specific cyclin-B1,G2/mitotic-specific cyclin-B1
| Macromolecule | Name: G2/mitotic-specific cyclin-B1,G2/mitotic-specific cyclin-B1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 52.625723 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MALRVTRNSK INAENKAKIN MAGAKRVPTA PAATSKPGLR PRTALGDIGN KVSEQLQAKM PMKKEAKPSA TGKVIDKKLP KPLEKVPML VPVPVSEPVP EPEPEPEPEP VKEEKLSPEP ILVDTASPSP METSGCAPAE EDLCQAFSDV ILAVNDVDAE D GADPNLCS ...String: MALRVTRNSK INAENKAKIN MAGAKRVPTA PAATSKPGLR PRTALGDIGN KVSEQLQAKM PMKKEAKPSA TGKVIDKKLP KPLEKVPML VPVPVSEPVP EPEPEPEPEP VKEEKLSPEP ILVDTASPSP METSGCAPAE EDLCQAFSDV ILAVNDVDAE D GADPNLCS EYVKDIYAYL RQLEEEQAVR PKYLLGREVT GNMRAILIDW LVQVQMKFRL LQETMYMTVS IIDRFMQNNC VP KKMLQLV GVTAMFIASK YEEMYPPEIG DFAFVTDNTY TKHQIRQMEM KILRALNFGL GRPLPLHFLR RASKIGEVDV EQH TLAKYL MELTMLDYDM VHFPPSQIAA GAFCLALKIL DNGEWTPTLQ HYLSYTEESL LPVMQHLAKN VVMVNQGLTK HMTV KNKYA TSKHAKISTL PQLNSALVQD LAKAVAKVSS LAEENLYFQS WSHPQFEKGG GSGGGSGGGS WSHPQFEK |
-Macromolecule #4: Cyclin-dependent kinases regulatory subunit 1
| Macromolecule | Name: Cyclin-dependent kinases regulatory subunit 1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 9.679211 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MSHKQIYYSD KYDDEEFEYR HVMLPKDIAK LVPKTHLMSE SEWRNLGVQQ SQGWVHYMIH EPEPHILLFR RPLPKKPKK |
-Macromolecule #5: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.050 mg/mL |
|---|---|
| Buffer | pH: 7.8 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: GRAPHENE OXIDE / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 293 K / Instrument: LEICA EM GP |
| Details | The sample was monodisperse. We use graphene oxide-coated EM grids. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 2.5 µm / Calibrated defocus min: 1.3 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.3 µm / Nominal magnification: 105000 Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.3 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 5 / Number real images: 13640 / Average exposure time: 3.0 sec. / Average electron dose: 78.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Software: (Name: Gctf, cryoSPARC) |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION / Number images used: 312836 |
 Movie
Movie Controller
Controller






























 Z
Z Y
Y X
X