+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7m3f | ||||||
|---|---|---|---|---|---|---|---|
| Title | Asymmetric Activation of the Calcium Sensing Receptor Homodimer | ||||||
 Components Components | Extracellular calcium-sensing receptor | ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  GPCR / GPCR /  calcium sensing receptor / active state / calcium sensing receptor / active state /  positive allosteric modulator / family C GPCR positive allosteric modulator / family C GPCR | ||||||
| Function / homology |  Function and homology information Function and homology informationbile acid secretion / chemosensory behavior / cellular response to peptide / response to fibroblast growth factor / cellular response to vitamin D / phosphatidylinositol phospholipase C activity / Class C/3 (Metabotropic glutamate/pheromone receptors) / calcium ion import / positive regulation of positive chemotaxis / fat pad development ...bile acid secretion / chemosensory behavior / cellular response to peptide / response to fibroblast growth factor / cellular response to vitamin D / phosphatidylinositol phospholipase C activity / Class C/3 (Metabotropic glutamate/pheromone receptors) / calcium ion import / positive regulation of positive chemotaxis / fat pad development /  amino acid binding / cellular response to hepatocyte growth factor stimulus / branching morphogenesis of an epithelial tube / positive regulation of calcium ion import / regulation of calcium ion transport / cellular response to low-density lipoprotein particle stimulus / detection of calcium ion / anatomical structure morphogenesis / axon terminus / JNK cascade / positive regulation of vasoconstriction / chloride transmembrane transport / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / amino acid binding / cellular response to hepatocyte growth factor stimulus / branching morphogenesis of an epithelial tube / positive regulation of calcium ion import / regulation of calcium ion transport / cellular response to low-density lipoprotein particle stimulus / detection of calcium ion / anatomical structure morphogenesis / axon terminus / JNK cascade / positive regulation of vasoconstriction / chloride transmembrane transport / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway /  ossification / response to ischemia / G protein-coupled receptor activity / cellular response to glucose stimulus / positive regulation of insulin secretion / intracellular calcium ion homeostasis / ossification / response to ischemia / G protein-coupled receptor activity / cellular response to glucose stimulus / positive regulation of insulin secretion / intracellular calcium ion homeostasis /  vasodilation / vasodilation /  integrin binding / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (i) signalling events / cellular response to hypoxia / G alpha (q) signalling events / basolateral plasma membrane / transmembrane transporter binding / positive regulation of ERK1 and ERK2 cascade / apical plasma membrane / G protein-coupled receptor signaling pathway / neuronal cell body / integrin binding / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (i) signalling events / cellular response to hypoxia / G alpha (q) signalling events / basolateral plasma membrane / transmembrane transporter binding / positive regulation of ERK1 and ERK2 cascade / apical plasma membrane / G protein-coupled receptor signaling pathway / neuronal cell body /  calcium ion binding / positive regulation of cell population proliferation / positive regulation of gene expression / calcium ion binding / positive regulation of cell population proliferation / positive regulation of gene expression /  protein kinase binding / protein kinase binding /  cell surface / protein homodimerization activity / identical protein binding / cell surface / protein homodimerization activity / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.8 Å cryo EM / Resolution: 2.8 Å | ||||||
 Authors Authors | Gao, Y. / Robertson, M.J. / Zhang, C. / Meyerowitz, J.G. / Panova, O. / Skiniotis, G. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Asymmetric activation of the calcium-sensing receptor homodimer. Authors: Yang Gao / Michael J Robertson / Sabrina N Rahman / Alpay B Seven / Chensong Zhang / Justin G Meyerowitz / Ouliana Panova / Fadil M Hannan / Rajesh V Thakker / Hans Bräuner-Osborne / Jesper ...Authors: Yang Gao / Michael J Robertson / Sabrina N Rahman / Alpay B Seven / Chensong Zhang / Justin G Meyerowitz / Ouliana Panova / Fadil M Hannan / Rajesh V Thakker / Hans Bräuner-Osborne / Jesper M Mathiesen / Georgios Skiniotis /    Abstract: The calcium-sensing receptor (CaSR), a cell-surface sensor for Ca, is the master regulator of calcium homeostasis in humans and is the target of calcimimetic drugs for the treatment of parathyroid ...The calcium-sensing receptor (CaSR), a cell-surface sensor for Ca, is the master regulator of calcium homeostasis in humans and is the target of calcimimetic drugs for the treatment of parathyroid disorders. CaSR is a family C G-protein-coupled receptor that functions as an obligate homodimer, with each protomer composed of a Ca-binding extracellular domain and a seven-transmembrane-helix domain (7TM) that activates heterotrimeric G proteins. Here we present cryo-electron microscopy structures of near-full-length human CaSR in inactive or active states bound to Ca and various calcilytic or calcimimetic drug molecules. We show that, upon activation, the CaSR homodimer adopts an asymmetric 7TM configuration that primes one protomer for G-protein coupling. This asymmetry is stabilized by 7TM-targeting calcimimetic drugs adopting distinctly different poses in the two protomers, whereas the binding of a calcilytic drug locks CaSR 7TMs in an inactive symmetric configuration. These results provide a detailed structural framework for CaSR activation and the rational design of therapeutics targeting this receptor. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7m3f.cif.gz 7m3f.cif.gz | 298.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7m3f.ent.gz pdb7m3f.ent.gz | 245.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7m3f.json.gz 7m3f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/m3/7m3f https://data.pdbj.org/pub/pdb/validation_reports/m3/7m3f ftp://data.pdbj.org/pub/pdb/validation_reports/m3/7m3f ftp://data.pdbj.org/pub/pdb/validation_reports/m3/7m3f | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  23653MC  7m3eC  7m3gC  7m3jC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 101745.445 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: 1-16 is signaling sequence, 17-24 is FLAG epitope tag, 25-27 is an 3-alanine linker, the receptor sequence starts at residue Y28 that should be re-numbered to 20, and ends at V902 that ...Details: 1-16 is signaling sequence, 17-24 is FLAG epitope tag, 25-27 is an 3-alanine linker, the receptor sequence starts at residue Y28 that should be re-numbered to 20, and ends at V902 that should be re-numbered to 894. Source: (gene. exp.)   Homo sapiens (human) / Gene: CASR, GPRC2A, PCAR1 / Cell (production host): sf9 / Cell line (production host): Sf9 / Production host: Homo sapiens (human) / Gene: CASR, GPRC2A, PCAR1 / Cell (production host): sf9 / Cell line (production host): Sf9 / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: P41180 Spodoptera frugiperda (fall armyworm) / References: UniProt: P41180 |
|---|
-Sugars , 2 types, 12 molecules 
| #2: Polysaccharide |  / Mass: 424.401 Da / Num. of mol.: 3 / Mass: 424.401 Da / Num. of mol.: 3Source method: isolated from a genetically manipulated source #3: Sugar | ChemComp-NAG /  N-Acetylglucosamine N-Acetylglucosamine |
|---|
-Non-polymers , 4 types, 10 molecules 
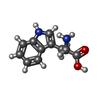





| #4: Chemical |  Cinacalcet Cinacalcet#5: Chemical |  Tryptophan Tryptophan#6: Chemical | ChemComp-CA / #7: Chemical |  Phosphate Phosphate |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: active-state human extracellular calcium-sensing receptor complexed with positive allosteric modulators etelcalcetide and evocalcet Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 200 kDa/nm / Experimental value: NO |
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 7 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / C2 aperture diameter: 70 µm Bright-field microscopy / C2 aperture diameter: 70 µm |
| Image recording | Electron dose: 64.9 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.18.2_3874: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
CTF correction | Type: NONE | ||||||||||||||||||||||||
3D reconstruction | Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 253836 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Highest resolution: 2.8 Å | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj







