[English] 日本語
 Yorodumi
Yorodumi- PDB-7bkc: Formate dehydrogenase - heterodisulfide reductase - formylmethano... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7bkc | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Formate dehydrogenase - heterodisulfide reductase - formylmethanofuran dehydrogenase complex from Methanospirillum hungatei (dimeric, composite structure) | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords |  OXIDOREDUCTASE / OXIDOREDUCTASE /  methanogenesis / flavin-based electron bifurcation / CO2-fixation / methanogenesis / flavin-based electron bifurcation / CO2-fixation /  formate dehydrogenase formate dehydrogenase | |||||||||||||||
| Function / homology |  Function and homology information Function and homology information formylmethanofuran dehydrogenase / formylmethanofuran dehydrogenase /  formylmethanofuran dehydrogenase / formylmethanofuran dehydrogenase /  formylmethanofuran dehydrogenase activity / formylmethanofuran dehydrogenase activity /  Oxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors / Oxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors /  CoB--CoM heterodisulfide reductase activity / CoB--CoM heterodisulfide reductase activity /  methanogenesis, from carbon dioxide / formate metabolic process / methanogenesis, from carbon dioxide / formate metabolic process /  Oxidoreductases; Acting on a sulfur group of donors / Oxidoreductases; Acting on a sulfur group of donors /  methanogenesis / formate dehydrogenase (NAD+) activity ... methanogenesis / formate dehydrogenase (NAD+) activity ... formylmethanofuran dehydrogenase / formylmethanofuran dehydrogenase /  formylmethanofuran dehydrogenase / formylmethanofuran dehydrogenase /  formylmethanofuran dehydrogenase activity / formylmethanofuran dehydrogenase activity /  Oxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors / Oxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors /  CoB--CoM heterodisulfide reductase activity / CoB--CoM heterodisulfide reductase activity /  methanogenesis, from carbon dioxide / formate metabolic process / methanogenesis, from carbon dioxide / formate metabolic process /  Oxidoreductases; Acting on a sulfur group of donors / Oxidoreductases; Acting on a sulfur group of donors /  methanogenesis / formate dehydrogenase (NAD+) activity / hydrolase activity, acting on carbon-nitrogen (but not peptide) bonds / molybdopterin cofactor binding / methanogenesis / formate dehydrogenase (NAD+) activity / hydrolase activity, acting on carbon-nitrogen (but not peptide) bonds / molybdopterin cofactor binding /  iron-sulfur cluster binding / transition metal ion binding / 4 iron, 4 sulfur cluster binding / iron-sulfur cluster binding / transition metal ion binding / 4 iron, 4 sulfur cluster binding /  oxidoreductase activity / oxidoreductase activity /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||||||||
| Biological species |   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea) | |||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3 Å cryo EM / Resolution: 3 Å | |||||||||||||||
 Authors Authors | Pfeil-Gardiner, O. / Watanabe, T. / Shima, S. / Murphy, B.J. | |||||||||||||||
| Funding support |  Germany, Germany,  Japan, 4items Japan, 4items
| |||||||||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Three-megadalton complex of methanogenic electron-bifurcating and CO-fixing enzymes. Authors: Tomohiro Watanabe / Olivia Pfeil-Gardiner / Jörg Kahnt / Jürgen Koch / Seigo Shima / Bonnie J Murphy /  Abstract: The first reaction of the methanogenic pathway from carbon dioxide (CO) is the reduction and condensation of CO to formyl-methanofuran, catalyzed by formyl-methanofuran dehydrogenase (Fmd). Strongly ...The first reaction of the methanogenic pathway from carbon dioxide (CO) is the reduction and condensation of CO to formyl-methanofuran, catalyzed by formyl-methanofuran dehydrogenase (Fmd). Strongly reducing electrons for this reaction are generated by heterodisulfide reductase (Hdr) in complex with hydrogenase or formate dehydrogenase (Fdh) using a flavin-based electron-bifurcation mechanism. Here, we report enzymological and structural characterizations of Fdh-Hdr-Fmd complexes from . The complexes catalyze this reaction using electrons from formate and the reduced form of the electron carrier F. Conformational changes in HdrA mediate electron bifurcation, and polyferredoxin FmdF directly transfers electrons to the CO reduction site, as evidenced by methanofuran-dependent flavin-based electron bifurcation even without free ferredoxin, a diffusible electron carrier between Hdr and Fmd. Conservation of Hdr and Fmd structures suggests that this complex is common among hydrogenotrophic methanogens. #1:  Journal: Comput. Cryst. Newsl. / Year: 2013 Journal: Comput. Cryst. Newsl. / Year: 2013Title: New tool: phenix.real_space_refine Authors: Afonine, P.V. / Headd, J.J. / Terwilliger, T.C. / Adams, P.D. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7bkc.cif.gz 7bkc.cif.gz | 1.4 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7bkc.ent.gz pdb7bkc.ent.gz | 1.2 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7bkc.json.gz 7bkc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bk/7bkc https://data.pdbj.org/pub/pdb/validation_reports/bk/7bkc ftp://data.pdbj.org/pub/pdb/validation_reports/bk/7bkc ftp://data.pdbj.org/pub/pdb/validation_reports/bk/7bkc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  12209MC  7bkbC  7bkdC  7bkeC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 5 types, 10 molecules AaFfEeDdIi
| #1: Protein | Mass: 72885.062 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FKZ1,  Oxidoreductases; Acting on a sulfur group of donors Oxidoreductases; Acting on a sulfur group of donors#2: Protein | Mass: 15692.425 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FKZ0 Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FKZ0#3: Protein |  Mass: 45639.895 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FME3,  Oxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors Oxidoreductases; Acting on the aldehyde or oxo group of donors; With unknown physiological acceptors#6: Protein |  Mass: 75911.445 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FRK1, Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FRK1,  formate dehydrogenase formate dehydrogenase#7: Protein |  Mass: 28696.902 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FRL8,  formylmethanofuran dehydrogenase formylmethanofuran dehydrogenase |
|---|
-CoB--CoM heterodisulfide reductase subunit ... , 2 types, 4 molecules CcBb
| #4: Protein |  CoB—CoM heterodisulfide reductase CoB—CoM heterodisulfide reductaseMass: 21706.963 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FKZ3 Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FKZ3#5: Protein |  CoB—CoM heterodisulfide reductase CoB—CoM heterodisulfide reductaseMass: 32930.938 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FKZ2 Methanospirillum hungatei JF-1 (archaea) / References: UniProt: Q2FKZ2 |
|---|
-Formylmethanofuran dehydrogenase, subunit ... , 5 types, 12 molecules LlGgJjMKmkHh
| #8: Protein |  Mass: 16388.020 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FKZ5,  formylmethanofuran dehydrogenase formylmethanofuran dehydrogenase#9: Protein |  Mass: 63636.211 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FRL9,  formylmethanofuran dehydrogenase formylmethanofuran dehydrogenase#10: Protein |  Mass: 15157.499 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FRF6,  formylmethanofuran dehydrogenase formylmethanofuran dehydrogenase#11: Protein |  Mass: 42216.734 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FKZ4,  formylmethanofuran dehydrogenase formylmethanofuran dehydrogenase#12: Protein |  Mass: 49371.586 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Methanospirillum hungatei JF-1 (archaea) Methanospirillum hungatei JF-1 (archaea)References: UniProt: Q2FRM0,  formylmethanofuran dehydrogenase formylmethanofuran dehydrogenase |
|---|
-Non-polymers , 7 types, 68 molecules 


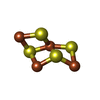









| #13: Chemical | ChemComp-SF4 /  Iron–sulfur cluster Iron–sulfur cluster#14: Chemical | ChemComp-FAD /  Flavin adenine dinucleotide Flavin adenine dinucleotide#15: Chemical |  Iron–sulfur cluster Iron–sulfur cluster#16: Chemical | ChemComp-9S8 / #17: Chemical | ChemComp-ZN / #18: Chemical | #19: Chemical | ChemComp-MGD / |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Dimeric formate dehydrogenase - heterodisulfide reductase - formylmethanofuran dehydrogenase complex Type: COMPLEX / Entity ID: #1-#12 / Source: NATURAL |
|---|---|
| Molecular weight | Value: 0.948 MDa / Experimental value: NO |
| Source (natural) | Organism:   Methanospirillum hungatei JF-1 (archaea) / Cellular location: cytoplasm Methanospirillum hungatei JF-1 (archaea) / Cellular location: cytoplasm |
| Buffer solution | pH: 7.6 |
| Buffer component | Conc.: 25 mM / Name: Tris-HCl Tris / Formula: Tris-HCl Tris / Formula: Tris-HCl Tris Tris |
| Specimen | Conc.: 0.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YESDetails: Preparation in an anaerobic tent (O2 < 20 ppm at all times, nearly always < 2ppm) |
| Specimen support | Details: 15mA / Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: C-flat-2/1 |
Vitrification | Instrument: HOMEMADE PLUNGER / Cryogen name: ETHANE / Humidity: 70 % / Chamber temperature: 293 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Cs Bright-field microscopy / Cs : 2.7 mm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE : 2.7 mm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of real images: 8745 |
| EM imaging optics | Energyfilter name : GIF Bioquantum / Energyfilter slit width: 20 eV : GIF Bioquantum / Energyfilter slit width: 20 eV |
- Processing
Processing
| Software | Name: PHENIX / Classification: refinement | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||
| Image processing | Details: Recorded in counted mode | ||||||||||||||||||||||||||||||||
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C2 (2 fold cyclic : C2 (2 fold cyclic ) ) | ||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 1239454 Details: As this is a composite map, the resolution is estimated from the resolutions for masked refinements of several regions, which range from 2.6 to 3.7 A. Symmetry type: POINT | ||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: OTHER | ||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: THROUGHOUT | ||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 96.67 Å2 / Biso mean: 46.8325 Å2 / Biso min: 6.51 Å2 | ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj






















