[English] 日本語
 Yorodumi
Yorodumi- EMDB-36593: Small molecule agonist (PCO371) bound to human parathyroid hormon... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Small molecule agonist (PCO371) bound to human parathyroid hormone receptor type 1 (PTH1R) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Class B /  GPCR / GPCR /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information parathyroid hormone receptor activity / G protein-coupled peptide receptor activity / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / parathyroid hormone receptor activity / G protein-coupled peptide receptor activity / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process /  bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger /  peptide hormone binding / chondrocyte differentiation / cell maturation ... peptide hormone binding / chondrocyte differentiation / cell maturation ... parathyroid hormone receptor activity / G protein-coupled peptide receptor activity / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / parathyroid hormone receptor activity / G protein-coupled peptide receptor activity / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process /  bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger /  peptide hormone binding / chondrocyte differentiation / cell maturation / peptide hormone binding / chondrocyte differentiation / cell maturation /  bone resorption / bone resorption /  skeletal system development / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / intracellular calcium ion homeostasis / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding / Inactivation, recovery and regulation of the phototransduction cascade / skeletal system development / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / intracellular calcium ion homeostasis / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding / Inactivation, recovery and regulation of the phototransduction cascade /  heterotrimeric G-protein complex / heterotrimeric G-protein complex /  : / G alpha (12/13) signalling events / : / G alpha (12/13) signalling events /  extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye /  GTPase binding / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (i) signalling events / fibroblast proliferation / G alpha (s) signalling events / G alpha (q) signalling events / basolateral plasma membrane / Ras protein signal transduction / in utero embryonic development / cell population proliferation / Extra-nuclear estrogen signaling / cell surface receptor signaling pathway / GTPase binding / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (i) signalling events / fibroblast proliferation / G alpha (s) signalling events / G alpha (q) signalling events / basolateral plasma membrane / Ras protein signal transduction / in utero embryonic development / cell population proliferation / Extra-nuclear estrogen signaling / cell surface receptor signaling pathway /  receptor complex / apical plasma membrane / G protein-coupled receptor signaling pathway / lysosomal membrane / negative regulation of cell population proliferation / receptor complex / apical plasma membrane / G protein-coupled receptor signaling pathway / lysosomal membrane / negative regulation of cell population proliferation /  GTPase activity / GTPase activity /  synapse / positive regulation of cell population proliferation / protein-containing complex binding / synapse / positive regulation of cell population proliferation / protein-containing complex binding /  signal transduction / protein homodimerization activity / extracellular exosome / signal transduction / protein homodimerization activity / extracellular exosome /  membrane / membrane /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.57 Å cryo EM / Resolution: 2.57 Å | |||||||||
 Authors Authors | Zhao L / He Q / Yuan Q / Gu Y / He X / Shan H / Li J / Wang K / Li Y / Hu W ...Zhao L / He Q / Yuan Q / Gu Y / He X / Shan H / Li J / Wang K / Li Y / Hu W / Wu K / Shen J / Xu HE | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Conserved class B GPCR activation by a biased intracellular agonist. Authors: Li-Hua Zhao / Qian He / Qingning Yuan / Yimin Gu / Xinheng He / Hong Shan / Junrui Li / Kai Wang / Yang Li / Wen Hu / Kai Wu / Jianhua Shen / H Eric Xu /  Abstract: Class B G-protein-coupled receptors (GPCRs), including glucagon-like peptide 1 receptor (GLP1R) and parathyroid hormone 1 receptor (PTH1R), are important drug targets. Injectable peptide drugs ...Class B G-protein-coupled receptors (GPCRs), including glucagon-like peptide 1 receptor (GLP1R) and parathyroid hormone 1 receptor (PTH1R), are important drug targets. Injectable peptide drugs targeting these receptors have been developed, but orally available small-molecule drugs remain under development. Here we report the high-resolution structure of human PTH1R in complex with the stimulatory G protein (G) and a small-molecule agonist, PCO371, which reveals an unexpected binding mode of PCO371 at the cytoplasmic interface of PTH1R with G. The PCO371-binding site is totally different from all binding sites previously reported for small molecules or peptide ligands in GPCRs. The residues that make up the PCO371-binding pocket are conserved in class B GPCRs, and a single alteration in PTH2R and two residue alterations in GLP1R convert these receptors to respond to PCO371. Functional assays reveal that PCO371 is a G-protein-biased agonist that is defective in promoting PTH1R-mediated arrestin signalling. Together, these results uncover a distinct binding site for designing small-molecule agonists for PTH1R and possibly other members of the class B GPCRs and define a receptor conformation that is specific only for G-protein activation but not arrestin signalling. These insights should facilitate the design of distinct types of class B GPCR small-molecule agonist for various therapeutic indications. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36593.map.gz emd_36593.map.gz | 5.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36593-v30.xml emd-36593-v30.xml emd-36593.xml emd-36593.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36593.png emd_36593.png | 103.6 KB | ||
| Filedesc metadata |  emd-36593.cif.gz emd-36593.cif.gz | 6.6 KB | ||
| Others |  emd_36593_half_map_1.map.gz emd_36593_half_map_1.map.gz emd_36593_half_map_2.map.gz emd_36593_half_map_2.map.gz | 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36593 http://ftp.pdbj.org/pub/emdb/structures/EMD-36593 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36593 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36593 | HTTPS FTP |
-Related structure data
| Related structure data |  8jr9MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36593.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36593.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.824 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_36593_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_36593_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : A conserved and druggable pocket in class B G protein coupled rec...
| Entire | Name: A conserved and druggable pocket in class B G protein coupled receptors for 2 orally active small molecule agonists |
|---|---|
| Components |
|
-Supramolecule #1: A conserved and druggable pocket in class B G protein coupled rec...
| Supramolecule | Name: A conserved and druggable pocket in class B G protein coupled receptors for 2 orally active small molecule agonists type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 160 KDa |
-Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.879465 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RATHRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RATHRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTSGIFETK FQVDKVNFHM FDVGAQRDER RKWIQCFNDV TAIIFVVDSS DYNRLQEALN DF KSIWNNR WLRTISVILF LNKQDLLAEK VLAGKSKIED YFPEFARYTT PEDATPEPGE DPRVTRAKYF IRDEFLRIST ASG DGRHYC YPHFTCSVDT ENARRIFNDC RDIIQRMHLR QYELL |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.330793 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: GSLLQSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQD GKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY LSCCRFLDDN Q IVTSSGDT ...String: GSLLQSELDQ LRQEAEQLKN QIRDARKACA DATLSQITNN IDPVGRIQMR TRRTLRGHLA KIYAMHWGTD SRLLVSASQD GKLIIWDSY TTNKVHAIPL RSSWVMTCAY APSGNYVACG GLDNICSIYN LKTREGNVRV SRELAGHTGY LSCCRFLDDN Q IVTSSGDT TCALWDIETG QQTTTFTGHT GDVMSLSLAP DTRLFVSGAC DASAKLWDVR EGMCRQTFTG HESDINAICF FP NGNAFAT GSDDATCRLF DLRADQELMT YSHDNIICGI TSVSFSKSGR LLLAGYDDFN CNVWDALKAD RAGVLAGHDN RVS CLGVTD DGMAVATGSW DSFLKIWNGS SGGGGS UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Nanobody 35
| Macromolecule | Name: Nanobody 35 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 15.343019 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MAQVQLQESG GGLVQPGGSL RLSCAASGFT FSNYKMNWVR QAPGKGLEWV SDISQSGASI SYTGSVKGRF TISRDNAKNT LYLQMNSLK PEDTAVYYCA RCPAPFTRDC FDVTSTTYAY RGQGTQVTVS SHHHHHHEPE A |
-Macromolecule #5: Parathyroid hormone/parathyroid hormone-related peptide receptor
| Macromolecule | Name: Parathyroid hormone/parathyroid hormone-related peptide receptor type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.78302 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: DADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASGK LYPESEEDKE APTGSRYRGR PCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE LVPGHNRTWA NYSECVKFLT NETREREVFD R LAMIYTVG ...String: DADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASGK LYPESEEDKE APTGSRYRGR PCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE LVPGHNRTWA NYSECVKFLT NETREREVFD R LAMIYTVG YSVSLASLTV AVLILAYFRR LHCTRNYIHM HLFLSFMLRA VSIFVKDAVL YSGATLDEAE RLTEEELRAI AQ APPPPAT AAAGYAGCRV AVTFFLYFLA TNYYWILVEG LYLHSLIFMA FFSEKKYLWG FTVFGWGLPA VFVAVWVSVR ATL ANTGCW DLSSGNKKWI IQVPILASIV LNFILFINIV RVLATKLRET NAGRCDTRQQ YRKLLKSTLV LMPLFGVHYI VFMA TPYTE VSGTLWQVQM HYEMLFNSFQ GFFVAIIYCF CNGEVQAEIK KSWSRWTLAL DFRRKARSGS SSYSYGPMVS HEF UniProtKB: Parathyroid hormone/parathyroid hormone-related peptide receptor |
-Macromolecule #6: PCO-371
| Macromolecule | Name: PCO-371 / type: ligand / ID: 6 / Number of copies: 1 / Formula: KHF |
|---|---|
| Molecular weight | Theoretical: 635.654 Da |
| Chemical component information | 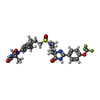 ChemComp-KHF: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.04 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 18.0 µm / Nominal defocus min: 8.0 µm Bright-field microscopy / Nominal defocus max: 18.0 µm / Nominal defocus min: 8.0 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.57 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 1099315 |
 Movie
Movie Controller
Controller




















 Z
Z Y
Y X
X

















