+ Open data
Open data
- Basic information
Basic information
| Entry | 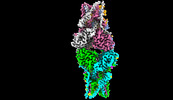 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Stimulator of interferon genes | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | adaptor protein /  IMMUNE SYSTEM IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationSTING mediated induction of host immune responses / 2',3'-cyclic GMP-AMP binding /  proton channel activity / cyclic-di-GMP binding / cGAS/STING signaling pathway / reticulophagy / autophagosome membrane / positive regulation of macroautophagy / proton channel activity / cyclic-di-GMP binding / cGAS/STING signaling pathway / reticulophagy / autophagosome membrane / positive regulation of macroautophagy /  autophagosome assembly / positive regulation of type I interferon production ...STING mediated induction of host immune responses / 2',3'-cyclic GMP-AMP binding / autophagosome assembly / positive regulation of type I interferon production ...STING mediated induction of host immune responses / 2',3'-cyclic GMP-AMP binding /  proton channel activity / cyclic-di-GMP binding / cGAS/STING signaling pathway / reticulophagy / autophagosome membrane / positive regulation of macroautophagy / proton channel activity / cyclic-di-GMP binding / cGAS/STING signaling pathway / reticulophagy / autophagosome membrane / positive regulation of macroautophagy /  autophagosome assembly / positive regulation of type I interferon production / autophagosome assembly / positive regulation of type I interferon production /  autophagosome / endoplasmic reticulum-Golgi intermediate compartment membrane / activation of innate immune response / positive regulation of interferon-beta production / Neutrophil degranulation / protein complex oligomerization / cytoplasmic vesicle / defense response to virus / autophagosome / endoplasmic reticulum-Golgi intermediate compartment membrane / activation of innate immune response / positive regulation of interferon-beta production / Neutrophil degranulation / protein complex oligomerization / cytoplasmic vesicle / defense response to virus /  Golgi membrane / Golgi membrane /  innate immune response / endoplasmic reticulum membrane / perinuclear region of cytoplasm / protein homodimerization activity / innate immune response / endoplasmic reticulum membrane / perinuclear region of cytoplasm / protein homodimerization activity /  metal ion binding / metal ion binding /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Gallus gallus (chicken) / Gallus gallus (chicken) /   Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria) Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.3 Å cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Lu DF / Shang GJ | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: The mechanism of STING autoinhibition and activation. Authors: Sheng Liu / Bo Yang / Yingxiang Hou / Kaige Cui / Xiaozhu Yang / Xiaoxiong Li / Lianwan Chen / Shichao Liu / Zhichao Zhang / Yuanyuan Jia / Yufeng Xie / Ying Xue / Xiaomei Li / Bingxue Yan / ...Authors: Sheng Liu / Bo Yang / Yingxiang Hou / Kaige Cui / Xiaozhu Yang / Xiaoxiong Li / Lianwan Chen / Shichao Liu / Zhichao Zhang / Yuanyuan Jia / Yufeng Xie / Ying Xue / Xiaomei Li / Bingxue Yan / Changxin Wu / Wen Deng / Jianxun Qi / Defen Lu / George F Gao / Peiyi Wang / Guijun Shang /  Abstract: 2',3'-cGAMP, produced by the DNA sensor cGAS, activates stimulator of interferon genes (STING) and triggers immune response during infection. Tremendous effort has been placed on unraveling the ...2',3'-cGAMP, produced by the DNA sensor cGAS, activates stimulator of interferon genes (STING) and triggers immune response during infection. Tremendous effort has been placed on unraveling the mechanism of STING activation. However, little is known about STING inhibition. Here, we found that apo-STING exhibits a bilayer with head-to-head as well as side-by-side packing, mediated by its ligand-binding domain (LBD). This type of assembly holds two endoplasmic reticulum (ER) membranes together not only to prevent STING ER exit but also to eliminate the recruitment of TBK1, representing the autoinhibited state of STING. Additionally, we obtained the filament structure of the STING/2',3'-cGAMP complex, which adopts a bent monolayer assembly mediated by LBD and transmembrane domain (TMD). The active, curved STING polymer could deform ER membrane to support its ER exit and anterograde transportation. Our data together provide a panoramic vision regarding STING autoinhibition and activation, which adds substantially to current understanding of the cGAS-STING pathway. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35503.map.gz emd_35503.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35503-v30.xml emd-35503-v30.xml emd-35503.xml emd-35503.xml | 14.3 KB 14.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35503.png emd_35503.png | 39.6 KB | ||
| Filedesc metadata |  emd-35503.cif.gz emd-35503.cif.gz | 5.4 KB | ||
| Others |  emd_35503_half_map_1.map.gz emd_35503_half_map_1.map.gz emd_35503_half_map_2.map.gz emd_35503_half_map_2.map.gz | 58.3 MB 58.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35503 http://ftp.pdbj.org/pub/emdb/structures/EMD-35503 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35503 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35503 | HTTPS FTP |
-Related structure data
| Related structure data |  8ik0MC  8ik3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35503.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35503.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.095 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_35503_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_35503_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : fiber of adaptor protein
| Entire | Name: fiber of adaptor protein |
|---|---|
| Components |
|
-Supramolecule #1: fiber of adaptor protein
| Supramolecule | Name: fiber of adaptor protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Gallus gallus (chicken) Gallus gallus (chicken) |
-Macromolecule #1: Stimulator of interferon genes protein,Immune protein Tsi3
| Macromolecule | Name: Stimulator of interferon genes protein,Immune protein Tsi3 type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria) Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) (bacteria) |
| Molecular weight | Theoretical: 55.282332 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MPQDPSTRSS PARLLIPEPR AGRARHAACV LLAVCFVVLF LSGEPLAPIT RRVCTQLAAL QLGVLLKGCC CLAEEIFHLH SRHHGSLWQ VLCSCFPPRW HLALLLVGGS AYLDPPEDNG HSPRLALTLS CLCQLLVLAL GLQKLSAVEV SELTESSKKN V AHGLAWSY ...String: MPQDPSTRSS PARLLIPEPR AGRARHAACV LLAVCFVVLF LSGEPLAPIT RRVCTQLAAL QLGVLLKGCC CLAEEIFHLH SRHHGSLWQ VLCSCFPPRW HLALLLVGGS AYLDPPEDNG HSPRLALTLS CLCQLLVLAL GLQKLSAVEV SELTESSKKN V AHGLAWSY YIGYLKVVLP RLKECMEEIS RTNPMLRAHR DTWKLHILVP LGCDIWDDLE KADSNIQYLA DLPETILTRA GI KRRVYKH SLYVIRDKDN KLRPCVLEFA SPLQTLCAMS QDDCAAFSRE QRLEQARLFY RSLRDILGSS KECAGLYRLI AYE EPAEPE SHFLSGLILW HLQQQQREEY MVLEVLFQGP VDFDKTLTHP NGLVVERPVG FDARRSAEGF RFDEGGKLRN PRQL EVQRQ DAPPPPDLAS RRLGDGEARY KVEEDDGGSA GSEYRLWAAK PAGARWIVVS ASEQSEDGEP TFALAWALLE RARLQ SSHH HHHHHH UniProtKB:  Stimulator of interferon genes protein, Stimulator of interferon genes protein,  Immune protein Tsi3 Immune protein Tsi3 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Details: 20mM HEPES, 150mM NaCl, 1mM TCEP, 0.03% DDM/CHS(5:1) |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: OTHER |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 1057869 |
 Movie
Movie Controller
Controller










 Z
Z Y
Y X
X


















