[English] 日本語
 Yorodumi
Yorodumi- EMDB-26256: Local refinement of cryo-EM structure of the interface of the Omi... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Local refinement of cryo-EM structure of the interface of the Omicron RBD in complex with antibodies B-182.1 and A19-46.1 | |||||||||
 Map data Map data | Local refinement map for the interface of Omicron RBD in complex with Fab B1-182.1 and Fab A19-46.1 | |||||||||
 Sample Sample |
| |||||||||
| Function / homology | Betacoronavirus spike (S) glycoprotein S1 subunit C-terminal (CTD) domain profile. / Spike (S) protein S1 subunit, receptor-binding domain, betacoronavirus / Spike S1 subunit, receptor binding domain superfamily, betacoronavirus / Betacoronavirus spike glycoprotein S1, receptor binding / Coronavirus spike glycoprotein S1, C-terminal / Coronavirus spike glycoprotein S1, C-terminal / Surface glycoprotein Function and homology information Function and homology information | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.8 Å cryo EM / Resolution: 4.8 Å | |||||||||
 Authors Authors | Zhou T / kwong PD | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B.1.1.529. Authors: Tongqing Zhou / Lingshu Wang / John Misasi / Amarendra Pegu / Yi Zhang / Darcy R Harris / Adam S Olia / Chloe Adrienna Talana / Eun Sung Yang / Man Chen / Misook Choe / Wei Shi / I-Ting Teng ...Authors: Tongqing Zhou / Lingshu Wang / John Misasi / Amarendra Pegu / Yi Zhang / Darcy R Harris / Adam S Olia / Chloe Adrienna Talana / Eun Sung Yang / Man Chen / Misook Choe / Wei Shi / I-Ting Teng / Adrian Creanga / Claudia Jenkins / Kwanyee Leung / Tracy Liu / Erik-Stephane D Stancofski / Tyler Stephens / Baoshan Zhang / Yaroslav Tsybovsky / Barney S Graham / John R Mascola / Nancy J Sullivan / Peter D Kwong /  Abstract: The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 (Omicron) variant and its resistance to neutralization by vaccinee and convalescent sera are driving a ...The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 (Omicron) variant and its resistance to neutralization by vaccinee and convalescent sera are driving a search for monoclonal antibodies with potent neutralization. To provide insight into effective neutralization, we determined cryo-electron microscopy structures and evaluated receptor binding domain (RBD) antibodies for their ability to bind and neutralize B.1.1.529. Mutations altered 16% of the B.1.1.529 RBD surface, clustered on an RBD ridge overlapping the angiotensin-converting enzyme 2 (ACE2)-binding surface and reduced binding of most antibodies. Substantial inhibitory activity was retained by select monoclonal antibodies-including A23-58.1, B1-182.1, COV2-2196, S2E12, A19-46.1, S309, and LY-CoV1404-that accommodated these changes and neutralized B.1.1.529. We identified combinations of antibodies with synergistic neutralization. The analysis revealed structural mechanisms for maintenance of potent neutralization against emerging variants. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26256.map.gz emd_26256.map.gz | 405.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26256-v30.xml emd-26256-v30.xml emd-26256.xml emd-26256.xml | 24.8 KB 24.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_26256.png emd_26256.png | 59.7 KB | ||
| Masks |  emd_26256_msk_1.map emd_26256_msk_1.map | 824 MB |  Mask map Mask map | |
| Others |  emd_26256_additional_1.map.gz emd_26256_additional_1.map.gz emd_26256_half_map_1.map.gz emd_26256_half_map_1.map.gz emd_26256_half_map_2.map.gz emd_26256_half_map_2.map.gz | 778.1 MB 765.4 MB 765.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26256 http://ftp.pdbj.org/pub/emdb/structures/EMD-26256 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26256 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26256 | HTTPS FTP |
-Related structure data
| Related structure data |  7u0dMC  7tb8C  7tbfC  7tc9C  7tcaC  7tccC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_26256.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26256.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement map for the interface of Omicron RBD in complex with Fab B1-182.1 and Fab A19-46.1 | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.855 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_26256_msk_1.map emd_26256_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
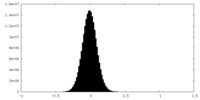
| Density Histograms |
-Additional map: Sharpened local refinement half map for the interface...
| File | emd_26256_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened local refinement half map for the interface of Omicron RBD in complex with Fab B1-182.1 and Fab A19-46.1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
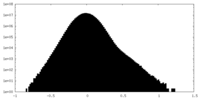
| Density Histograms |
-Half map: Local refinement half map for the interface of...
| File | emd_26256_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement half map for the interface of Omicron RBD in complex with Fab B1-182.1 and Fab A19-46.1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Local refinement half map for the interface of...
| File | emd_26256_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement half map for the interface of Omicron RBD in complex with Fab B1-182.1 and Fab A19-46.1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of SARS-CoV-2 Omicron RBD in complex with antibod...
| Entire | Name: Ternary complex of SARS-CoV-2 Omicron RBD in complex with antibodies B1-182.1 and A19-46.1 |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of SARS-CoV-2 Omicron RBD in complex with antibod...
| Supramolecule | Name: Ternary complex of SARS-CoV-2 Omicron RBD in complex with antibodies B1-182.1 and A19-46.1 type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Surface glycoprotein
| Macromolecule | Name: Surface glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Severe acute respiratory syndrome coronavirus 2 Severe acute respiratory syndrome coronavirus 2 |
| Molecular weight | Theoretical: 22.297182 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NITNLCPFDE VFNATRFASV YAWNRKRISN CVADYSVLYN LAPFFTFKCY GVSPTKLNDL CFTNVYADSF VIRGDEVRQI APGQTGNIA DYNYKLPDDF TGCVIAWNSN KLDSKVSGNY NYLYRLFRKS NLKPFERDIS TEIYQAGNKP CNGVAGFNCY F PLRSYSFR ...String: NITNLCPFDE VFNATRFASV YAWNRKRISN CVADYSVLYN LAPFFTFKCY GVSPTKLNDL CFTNVYADSF VIRGDEVRQI APGQTGNIA DYNYKLPDDF TGCVIAWNSN KLDSKVSGNY NYLYRLFRKS NLKPFERDIS TEIYQAGNKP CNGVAGFNCY F PLRSYSFR PTYGVGHQPY RVVVLSFELL HAPATVCG |
-Macromolecule #2: Heavy chain of SARS-CoV-2 antibody A19-46.1
| Macromolecule | Name: Heavy chain of SARS-CoV-2 antibody A19-46.1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.8699 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLVESGGG VVQPGRSLRL SCAASGFTLS SYGMHWVRQA PGKGLEWVAV ISYDGSNKYY VDSVKGRFTI SRDNSKNTLY LQMNSLRAE DTAVYYCARG WAYWELLPDY YYGMDVWGQG TTVTVSSAST KGPSVFPLAP SSKSTSGGTA ALGCLVKDYF P EPVTVSWN ...String: QVQLVESGGG VVQPGRSLRL SCAASGFTLS SYGMHWVRQA PGKGLEWVAV ISYDGSNKYY VDSVKGRFTI SRDNSKNTLY LQMNSLRAE DTAVYYCARG WAYWELLPDY YYGMDVWGQG TTVTVSSAST KGPSVFPLAP SSKSTSGGTA ALGCLVKDYF P EPVTVSWN SGALTSGVHT FPAVLQSSGL YSLSSVVTVP SSSLGTQTYI CNVNHKPSNT KVDKKVEPKS CDK |
-Macromolecule #3: Light chain of SARS-CoV-2 antibody A19-46.1
| Macromolecule | Name: Light chain of SARS-CoV-2 antibody A19-46.1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 22.760273 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QTVVTQEPSF SVSPGGTVTL TCGLSSGSVS TAYFPSWYQQ TPGQAPRTLI YGTNTRSSGV PDRFSGSILG NKAALTITGA QADDESDYY CVLYMGRGIV VFGGGTKLTV LGQPKAAPSV TLFPPSSEEL QANKATLVCL ISDFYPGAVT VAWKADSSPV K AGVETTTP ...String: QTVVTQEPSF SVSPGGTVTL TCGLSSGSVS TAYFPSWYQQ TPGQAPRTLI YGTNTRSSGV PDRFSGSILG NKAALTITGA QADDESDYY CVLYMGRGIV VFGGGTKLTV LGQPKAAPSV TLFPPSSEEL QANKATLVCL ISDFYPGAVT VAWKADSSPV K AGVETTTP SKQSNNKYAA SSYLSLTPEQ WKSHRSYSCQ VTHEGSTVEK TVAPTECS |
-Macromolecule #4: Heavy chain of SARS-CoV-2 antibody B1-182.1
| Macromolecule | Name: Heavy chain of SARS-CoV-2 antibody B1-182.1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.285312 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QMQLVQSGPE VKKPGTSVKV SCKASGFTFT SSAVQWVRQA RGQRLEWIGW IVVGSGNTNY AQKFQERVTI TRDMSTSTAY MELSSLRSE DTAVYYCAAP YCSGGSCFDG FDIWGQGTMV TVSSASTKGP SVFPLAPSSK STSGGTAALG CLVKDYFPEP V TVSWNSGA ...String: QMQLVQSGPE VKKPGTSVKV SCKASGFTFT SSAVQWVRQA RGQRLEWIGW IVVGSGNTNY AQKFQERVTI TRDMSTSTAY MELSSLRSE DTAVYYCAAP YCSGGSCFDG FDIWGQGTMV TVSSASTKGP SVFPLAPSSK STSGGTAALG CLVKDYFPEP V TVSWNSGA LTSGVHTFPA VLQSSGLYSL SSVVTVPSSS LGTQTYICNV NHKPSNTKVD KKVEPKSCDK |
-Macromolecule #5: Light chain of SARS-CoV-2 antibody B1-182.1
| Macromolecule | Name: Light chain of SARS-CoV-2 antibody B1-182.1 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.536008 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EIVLTQSPGT LSLSPGERAT LSCRASQSVS SSYLAWYQQK PGQAPRLLIY GASSRATGFP DRFSGSGSGT DFTLTISRLE PEDFAVYYC QQYGNSPWTF GQGTKVEIRR TVAAPSVFIF PPSDEQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD ...String: EIVLTQSPGT LSLSPGERAT LSCRASQSVS SSYLAWYQQK PGQAPRLLIY GASSRATGFP DRFSGSGSGT DFTLTISRLE PEDFAVYYC QQYGNSPWTF GQGTKVEIRR TVAAPSVFIF PPSDEQLKSG TASVVCLLNN FYPREAKVQW KVDNALQSGN S QESVTEQD SKDSTYSLSS TLTLSKADYE KHKVYACEVT HQGLSSPVTK SFNRGEC |
-Macromolecule #6: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 6 / Number of copies: 1 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 100 mM HEPES, 150 mM NaCl |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Blot for 2-3.5 seconds before plugging.. |
| Details | Complex at 0.5 mg/mL concentration in the buffer |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 46244 Details: SARS-CoV-2 receptor-binding domain in complex with antibodies B1-182.1 and A19-46.1 |
|---|---|
| CTF correction | Software - Name: cryoSPARC (ver. 3) |
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.8 Å / Resolution method: FSC 0.5 CUT-OFF / Number images used: 46244 |
 Movie
Movie Controller
Controller








 Z
Z Y
Y X
X