[English] 日本語
 Yorodumi
Yorodumi- EMDB-17924: Cryo-EM structure of human Elp123 in complex with tRNA, acetyl-Co... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human Elp123 in complex with tRNA, acetyl-CoA, 5'-deoxyadenosine and methionine | |||||||||
 Map data Map data | deepEMhancer sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Elongator / tRNA modification / acetyl-CoA hydrolysis /  TRANSLATION TRANSLATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationphosphorylase kinase regulator activity / tRNA uridine(34) acetyltransferase activity / elongator holoenzyme complex / tRNA wobble base 5-methoxycarbonylmethyl-2-thiouridinylation / tRNA wobble uridine modification / regulation of receptor signaling pathway via JAK-STAT /  acetyltransferase activity / acetyltransferase activity /  Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / transcription elongation factor complex / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / transcription elongation factor complex /  central nervous system development ...phosphorylase kinase regulator activity / tRNA uridine(34) acetyltransferase activity / elongator holoenzyme complex / tRNA wobble base 5-methoxycarbonylmethyl-2-thiouridinylation / tRNA wobble uridine modification / regulation of receptor signaling pathway via JAK-STAT / central nervous system development ...phosphorylase kinase regulator activity / tRNA uridine(34) acetyltransferase activity / elongator holoenzyme complex / tRNA wobble base 5-methoxycarbonylmethyl-2-thiouridinylation / tRNA wobble uridine modification / regulation of receptor signaling pathway via JAK-STAT /  acetyltransferase activity / acetyltransferase activity /  Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / transcription elongation factor complex / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / transcription elongation factor complex /  central nervous system development / transcription elongation by RNA polymerase II / central nervous system development / transcription elongation by RNA polymerase II /  neuron migration / neuron migration /  : / : /  regulation of translation / 4 iron, 4 sulfur cluster binding / HATs acetylate histones / regulation of translation / 4 iron, 4 sulfur cluster binding / HATs acetylate histones /  tRNA binding / positive regulation of cell migration / tRNA binding / positive regulation of cell migration /  nucleolus / regulation of transcription by RNA polymerase II / nucleolus / regulation of transcription by RNA polymerase II /  protein kinase binding / protein kinase binding /  nucleoplasm / nucleoplasm /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
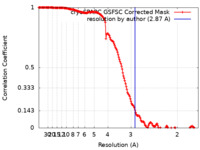
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.87 Å cryo EM / Resolution: 2.87 Å | |||||||||
 Authors Authors | Abbassi N / Jaciuk M / Lin T-Y / Glatt S | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM structure of human Elp123 in complex with tRNA, acetyl-CoA, 5'-deoxyadenosine and methionine Authors: Abbassi N / Jaciuk M / Lin T-Y / Glatt S | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17924.map.gz emd_17924.map.gz | 352.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17924-v30.xml emd-17924-v30.xml emd-17924.xml emd-17924.xml | 24.8 KB 24.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17924_fsc.xml emd_17924_fsc.xml | 15.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_17924.png emd_17924.png | 104.6 KB | ||
| Masks |  emd_17924_msk_1.map emd_17924_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17924.cif.gz emd-17924.cif.gz | 8.7 KB | ||
| Others |  emd_17924_half_map_1.map.gz emd_17924_half_map_1.map.gz emd_17924_half_map_2.map.gz emd_17924_half_map_2.map.gz | 392 MB 392 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17924 http://ftp.pdbj.org/pub/emdb/structures/EMD-17924 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17924 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17924 | HTTPS FTP |
-Related structure data
| Related structure data |  8ptxMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17924.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17924.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | deepEMhancer sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17924_msk_1.map emd_17924_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_17924_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17924_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Human Elp123 in complex with glutamine tRNA, acetyl-CoA, 5'-deoxy...
+Supramolecule #1: Human Elp123 in complex with glutamine tRNA, acetyl-CoA, 5'-deoxy...
+Macromolecule #1: Elongator complex protein 1
+Macromolecule #2: Elongator complex protein 2
+Macromolecule #3: Elongator complex protein 3
+Macromolecule #4: tRNA Gln
+Macromolecule #5: IRON/SULFUR CLUSTER
+Macromolecule #6: 5'-DEOXYADENOSINE
+Macromolecule #7: ACETYL COENZYME *A
+Macromolecule #8: METHIONINE
+Macromolecule #9: MAGNESIUM ION
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.6 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Details: 8 mA | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 15 s wait time, blot force 5, 5 s blot time. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000 |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number real images: 4214 / Average electron dose: 40.09 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Output model |  PDB-8ptx: |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)