[English] 日本語
 Yorodumi
Yorodumi- EMDB-17112: Structure of apo form of human gamma-secretase PSEN1 APH-1B isofo... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of apo form of human gamma-secretase PSEN1 APH-1B isoform reconstituted into lipid nanodisc | |||||||||||||||
 Map data Map data | Local resolution filtered masked map after pixel size calibration | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | intramembrane proteolysis /  protease / di-aspartyl protease / protease / di-aspartyl protease /  Alzheimer's disease / Alzheimer's disease /  complex / complex /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationCajal-Retzius cell differentiation / positive regulation of L-glutamate import across plasma membrane / amyloid precursor protein biosynthetic process / positive regulation of coagulation / protein catabolic process at postsynapse / negative regulation of core promoter binding /  gamma-secretase complex / aspartic endopeptidase activity, intramembrane cleaving / short-term synaptic potentiation / positive regulation of amyloid precursor protein biosynthetic process ...Cajal-Retzius cell differentiation / positive regulation of L-glutamate import across plasma membrane / amyloid precursor protein biosynthetic process / positive regulation of coagulation / protein catabolic process at postsynapse / negative regulation of core promoter binding / gamma-secretase complex / aspartic endopeptidase activity, intramembrane cleaving / short-term synaptic potentiation / positive regulation of amyloid precursor protein biosynthetic process ...Cajal-Retzius cell differentiation / positive regulation of L-glutamate import across plasma membrane / amyloid precursor protein biosynthetic process / positive regulation of coagulation / protein catabolic process at postsynapse / negative regulation of core promoter binding /  gamma-secretase complex / aspartic endopeptidase activity, intramembrane cleaving / short-term synaptic potentiation / positive regulation of amyloid precursor protein biosynthetic process / Noncanonical activation of NOTCH3 / positive regulation of endopeptidase activity / choline transport / Notch receptor processing / sequestering of calcium ion / gamma-secretase complex / aspartic endopeptidase activity, intramembrane cleaving / short-term synaptic potentiation / positive regulation of amyloid precursor protein biosynthetic process / Noncanonical activation of NOTCH3 / positive regulation of endopeptidase activity / choline transport / Notch receptor processing / sequestering of calcium ion /  central nervous system myelination / synaptic vesicle targeting / central nervous system myelination / synaptic vesicle targeting /  membrane protein intracellular domain proteolysis / negative regulation of axonogenesis / membrane protein intracellular domain proteolysis / negative regulation of axonogenesis /  regulation of resting membrane potential / T cell activation involved in immune response / NOTCH4 Activation and Transmission of Signal to the Nucleus / skin morphogenesis / regulation of resting membrane potential / T cell activation involved in immune response / NOTCH4 Activation and Transmission of Signal to the Nucleus / skin morphogenesis /  growth factor receptor binding / neural retina development / dorsal/ventral neural tube patterning / regulation of synaptic vesicle cycle / L-glutamate import across plasma membrane / myeloid dendritic cell differentiation / Regulated proteolysis of p75NTR / growth factor receptor binding / neural retina development / dorsal/ventral neural tube patterning / regulation of synaptic vesicle cycle / L-glutamate import across plasma membrane / myeloid dendritic cell differentiation / Regulated proteolysis of p75NTR /  regulation of phosphorylation / locomotion / brain morphogenesis / glutamate receptor signaling pathway / endoplasmic reticulum calcium ion homeostasis / nuclear outer membrane / smooth endoplasmic reticulum calcium ion homeostasis / amyloid precursor protein metabolic process / astrocyte activation involved in immune response / regulation of canonical Wnt signaling pathway / regulation of phosphorylation / locomotion / brain morphogenesis / glutamate receptor signaling pathway / endoplasmic reticulum calcium ion homeostasis / nuclear outer membrane / smooth endoplasmic reticulum calcium ion homeostasis / amyloid precursor protein metabolic process / astrocyte activation involved in immune response / regulation of canonical Wnt signaling pathway /  aggresome / regulation of long-term synaptic potentiation / embryonic limb morphogenesis / aggresome / regulation of long-term synaptic potentiation / embryonic limb morphogenesis /  skeletal system morphogenesis / cell fate specification / regulation of postsynapse organization / skeletal system morphogenesis / cell fate specification / regulation of postsynapse organization /  ciliary rootlet / myeloid cell homeostasis / azurophil granule membrane / dopamine receptor signaling pathway / ciliary rootlet / myeloid cell homeostasis / azurophil granule membrane / dopamine receptor signaling pathway /  Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / Golgi cisterna membrane / positive regulation of amyloid fibril formation / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / Golgi cisterna membrane / positive regulation of amyloid fibril formation /  adult behavior / positive regulation of receptor recycling / positive regulation of dendritic spine development / mitochondrial transport / positive regulation of catalytic activity / regulation of neuron projection development / heart looping / blood vessel development / amyloid precursor protein catabolic process / cerebral cortex cell migration / adult behavior / positive regulation of receptor recycling / positive regulation of dendritic spine development / mitochondrial transport / positive regulation of catalytic activity / regulation of neuron projection development / heart looping / blood vessel development / amyloid precursor protein catabolic process / cerebral cortex cell migration /  smooth endoplasmic reticulum / protein glycosylation / amyloid-beta formation / smooth endoplasmic reticulum / protein glycosylation / amyloid-beta formation /  autophagosome assembly / negative regulation of apoptotic signaling pathway / autophagosome assembly / negative regulation of apoptotic signaling pathway /  membrane protein ectodomain proteolysis / endopeptidase activator activity / EPH-ephrin mediated repulsion of cells / neuron development / membrane protein ectodomain proteolysis / endopeptidase activator activity / EPH-ephrin mediated repulsion of cells / neuron development /  rough endoplasmic reticulum / hematopoietic progenitor cell differentiation / rough endoplasmic reticulum / hematopoietic progenitor cell differentiation /  somitogenesis / amyloid-beta metabolic process / T cell proliferation / Nuclear signaling by ERBB4 / somitogenesis / amyloid-beta metabolic process / T cell proliferation / Nuclear signaling by ERBB4 /  Notch signaling pathway / negative regulation of ubiquitin-dependent protein catabolic process / Notch signaling pathway / negative regulation of ubiquitin-dependent protein catabolic process /  regulation of synaptic transmission, glutamatergic / regulation of synaptic transmission, glutamatergic /  transport vesicle / NOTCH2 Activation and Transmission of Signal to the Nucleus / cellular response to calcium ion / neuron projection maintenance / Degradation of the extracellular matrix / NRIF signals cell death from the nucleus / Activated NOTCH1 Transmits Signal to the Nucleus / positive regulation of glycolytic process / cerebellum development / post-embryonic development / thymus development / dendritic shaft / negative regulation of protein phosphorylation / epithelial cell proliferation / transport vesicle / NOTCH2 Activation and Transmission of Signal to the Nucleus / cellular response to calcium ion / neuron projection maintenance / Degradation of the extracellular matrix / NRIF signals cell death from the nucleus / Activated NOTCH1 Transmits Signal to the Nucleus / positive regulation of glycolytic process / cerebellum development / post-embryonic development / thymus development / dendritic shaft / negative regulation of protein phosphorylation / epithelial cell proliferation /  PDZ domain binding / NOTCH3 Activation and Transmission of Signal to the Nucleus / astrocyte activation / apoptotic signaling pathway / synapse organization PDZ domain binding / NOTCH3 Activation and Transmission of Signal to the Nucleus / astrocyte activation / apoptotic signaling pathway / synapse organizationSimilarity search - Function | |||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
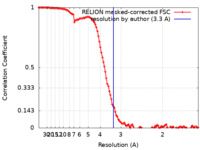
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.3 Å cryo EM / Resolution: 3.3 Å | |||||||||||||||
 Authors Authors | Odorcic I / Chavez Gutierrez L / Efremov RG | |||||||||||||||
| Funding support |  Belgium, 4 items Belgium, 4 items
| |||||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Complex of human APH-1B isoform of gamma-secretase with Amyloid-beta in lipid environment suggests substrate shifting as the mechanism of sequential cleavage Authors: Odorcic I | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17112.map.gz emd_17112.map.gz | 80.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17112-v30.xml emd-17112-v30.xml emd-17112.xml emd-17112.xml | 26.2 KB 26.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17112_fsc.xml emd_17112_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_17112.png emd_17112.png | 88.1 KB | ||
| Masks |  emd_17112_msk_1.map emd_17112_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17112.cif.gz emd-17112.cif.gz | 7.9 KB | ||
| Others |  emd_17112_additional_1.map.gz emd_17112_additional_1.map.gz emd_17112_half_map_1.map.gz emd_17112_half_map_1.map.gz emd_17112_half_map_2.map.gz emd_17112_half_map_2.map.gz | 116.7 MB 98.3 MB 98.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17112 http://ftp.pdbj.org/pub/emdb/structures/EMD-17112 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17112 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17112 | HTTPS FTP |
-Related structure data
| Related structure data |  8oqyMC  8oqzC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17112.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17112.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution filtered masked map after pixel size calibration | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.776 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17112_msk_1.map emd_17112_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map after pixel size calibration
| File | emd_17112_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map after pixel size calibration | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map (2)
| File | emd_17112_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map (2) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map (1)
| File | emd_17112_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map (1) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Gamma secretase
| Entire | Name: Gamma secretase |
|---|---|
| Components |
|
-Supramolecule #1: Gamma secretase
| Supramolecule | Name: Gamma secretase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 172 KDa |
-Macromolecule #1: Nicastrin
| Macromolecule | Name: Nicastrin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 79.371586 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MATAGGGSGA DPGSRGLLRL LSFCVLLAGL CRGNSVERKI YIPLNKTAPC VRLLNATHQI GCQSSISGDT GVIHVVEKEE DLQWVLTDG PNPPYMVLLE SKHFTRDLME KLKGRTSRIA GLAVSLTKPS PASGFSPSVQ CPNDGFGVYS NSYGPEFAHC R EIQWNSLG ...String: MATAGGGSGA DPGSRGLLRL LSFCVLLAGL CRGNSVERKI YIPLNKTAPC VRLLNATHQI GCQSSISGDT GVIHVVEKEE DLQWVLTDG PNPPYMVLLE SKHFTRDLME KLKGRTSRIA GLAVSLTKPS PASGFSPSVQ CPNDGFGVYS NSYGPEFAHC R EIQWNSLG NGLAYEDFSF PIFLLEDENE TKVIKQCYQD HNLSQNGSAP TFPLCAMQLF SHMHAVISTA TCMRRSSIQS TF SINPEIV CDPLSDYNVW SMLKPINTTG TLKPDDRVVV AATRLDSRSF FWNVAPGAES AVASFVTQLA AAEALQKAPD VTT LPRNVM FVFFQGETFD YIGSSRMVYD MEKGKFPVQL ENVDSFVELG QVALRTSLEL WMHTDPVSQK NESVRNQVED LLAT LEKSG AGVPAVILRR PNQSQPLPPS SLQRFLRARN ISGVVLADHS GAFHNKYYQS IYDTAENINV SYPEWLSPEE DLNFV TDTA KALADVATVL GRALYELAGG TNFSDTVQAD PQTVTRLLYG FLIKANNSWF QSILRQDLRS YLGDGPLQHY IAVSSP TNT TYVVQYALAN LTGTVVNLTR EQCQDPSKVP SENKDLYEYS WVQGPLHSNE TDRLPRCVRS TARLARALSP AFELSQW SS TEYSTWTESR WKDIRARIFL IASKELELIT LTVGFGILIF SLIVTYCINA KADVLFIAPR EPGAVSYGTL EVLFQ UniProtKB:  Nicastrin Nicastrin |
-Macromolecule #2: Presenilin-1 CTF12
| Macromolecule | Name: Presenilin-1 CTF12 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 52.713535 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MTELPAPLSY FQNAQMSEDN HLSNTVRSQN DNRERQEHND RRSLGHPEPL SNGRPQGNSR QVVEQDEEED EELTLKYGAK HVIMLFVPV TLCMVVVVAT IKSVSFYTRK DGQLIYTPFT EDTETVGQRA LHSILNAAIM ISVIVVMTIL LVVLYKYRCY K VIHAWLII ...String: MTELPAPLSY FQNAQMSEDN HLSNTVRSQN DNRERQEHND RRSLGHPEPL SNGRPQGNSR QVVEQDEEED EELTLKYGAK HVIMLFVPV TLCMVVVVAT IKSVSFYTRK DGQLIYTPFT EDTETVGQRA LHSILNAAIM ISVIVVMTIL LVVLYKYRCY K VIHAWLII SSLLLLFFFS FIYLGEVFKT YNVAVDYITV ALLIWNFGVV GMISIHWKGP LRLQQAYLIM ISALMALVFI KY LPEWTAW LILAVISVYD LVAVLCPKGP LRMLVETAQE RNETLFPALI YSSTMVWLVN MAEGDPEAQR RVSKNSKYNA EST ERESQD TVAENDDGGF SEEWEAQRDS HLGPHRSTPE SRAAVQELSS SILAGEDPEE RGVKLGLGDF IFYSVLVGKA SATA SGDWN TTIACFVAIL IGLCLTLLLL AIFKKALPAL PISITFGLVF YFATDYLVQP FMDQLAFHQF YI UniProtKB:  Presenilin-1 Presenilin-1 |
-Macromolecule #3: Gamma-secretase subunit APH-1B
| Macromolecule | Name: Gamma-secretase subunit APH-1B / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.480844 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MTAAVFFGCA FIAFGPALAL YVFTIATEPL RIIFLIAGAF FWLVSLLISS LVWFMARVII DNKDGPTQKY LLIFGAFVSV YIQEMFRFA YYKLLKKASE GLKSINPGET APSMRLLAYV SGLGFGIMSG VFSFVNTLSD SLGPGTVGIH GDSPQFFLYS A FMTLVIIL ...String: MTAAVFFGCA FIAFGPALAL YVFTIATEPL RIIFLIAGAF FWLVSLLISS LVWFMARVII DNKDGPTQKY LLIFGAFVSV YIQEMFRFA YYKLLKKASE GLKSINPGET APSMRLLAYV SGLGFGIMSG VFSFVNTLSD SLGPGTVGIH GDSPQFFLYS A FMTLVIIL LHVFWGIVFF DGCEKKKWGI LLIVLLTHLL VSAQTFISSY YGINLASAFI ILVLMGTWAF LAAGGSCRSL KL CLLCQDK NFLLYNQRSR UniProtKB:  Gamma-secretase subunit APH-1B Gamma-secretase subunit APH-1B |
-Macromolecule #4: Gamma-secretase subunit PEN-2
| Macromolecule | Name: Gamma-secretase subunit PEN-2 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.038029 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MNLERVSNEE KLNLCRKYYL GGFAFLPFLW LVNIFWFFRE AFLVPAYTEQ SQIKGYVWRS AVGFLFWVIV LTSWITIFQI YRPRWGALG DYLSFTIPLG TP UniProtKB:  Gamma-secretase subunit PEN-2 Gamma-secretase subunit PEN-2 |
-Macromolecule #7: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 7 / Number of copies: 7 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #8: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 8 / Number of copies: 3 / Formula: PC1 |
|---|---|
| Molecular weight | Theoretical: 790.145 Da |
| Chemical component information |  ChemComp-PC1: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.04 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||
| Grid | Model: Quantifoil R0.6/1 / Material: COPPER / Mesh: 300 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: GRAPHENE OXIDE / Support film - #1 - topology: CONTINUOUS | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 92 % / Chamber temperature: 296 K / Instrument: GATAN CRYOPLUNGE 3 |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 3.0 µm / Calibrated defocus min: 1.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.55 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 60000 Bright-field microscopy / Cs: 2.55 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 60000 |
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: JEOL CRYOSPECPORTER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 10733 / Average exposure time: 3.0 sec. / Average electron dose: 61.0 e/Å2 |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT | ||||||
| Output model |  PDB-8oqy: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)