+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10590 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
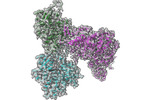
| Title | Structure of PfMyoA decorated Plasmodium Act1 filament | |||||||||
 Map data Map data | Global B-factor sharpening | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationplastid inheritance /  schizogony / pellicle / glideosome / inner membrane pellicle complex / Platelet degranulation / symbiont-medited actin polymerization-dependent cell-to-cell migration in host / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / Neutrophil degranulation / entry into host cell by a symbiont-containing vacuole ...plastid inheritance / schizogony / pellicle / glideosome / inner membrane pellicle complex / Platelet degranulation / symbiont-medited actin polymerization-dependent cell-to-cell migration in host / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / Neutrophil degranulation / entry into host cell by a symbiont-containing vacuole ...plastid inheritance /  schizogony / pellicle / glideosome / inner membrane pellicle complex / Platelet degranulation / symbiont-medited actin polymerization-dependent cell-to-cell migration in host / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / Neutrophil degranulation / entry into host cell by a symbiont-containing vacuole / vesicle transport along actin filament / schizogony / pellicle / glideosome / inner membrane pellicle complex / Platelet degranulation / symbiont-medited actin polymerization-dependent cell-to-cell migration in host / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / Neutrophil degranulation / entry into host cell by a symbiont-containing vacuole / vesicle transport along actin filament /  myosin complex / microfilament motor activity / cytoskeletal motor activity / cytoskeleton organization / actin filament organization / myosin complex / microfilament motor activity / cytoskeletal motor activity / cytoskeleton organization / actin filament organization /  actin filament / actin filament /  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of cytoskeleton / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of cytoskeleton /  actin filament binding / actin filament binding /  actin cytoskeleton / actin cytoskeleton /  actin binding / vesicle / actin binding / vesicle /  ATP hydrolysis activity / ATP hydrolysis activity /  ATP binding / ATP binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Plasmodium falciparum 3D7 (eukaryote) Plasmodium falciparum 3D7 (eukaryote) | |||||||||
| Method | helical reconstruction /  cryo EM / Resolution: 3.1 Å cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Vahokoski J / Calder LJ / Lopez AJ / Rosenthal PB / Kursula I | |||||||||
| Funding support |  Norway, Norway,  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2022 Journal: PLoS Pathog / Year: 2022Title: High-resolution structures of malaria parasite actomyosin and actin filaments. Authors: Juha Vahokoski / Lesley J Calder / Andrea J Lopez / Justin E Molloy / Inari Kursula / Peter B Rosenthal /    Abstract: Malaria is responsible for half a million deaths annually and poses a huge economic burden on the developing world. The mosquito-borne parasites (Plasmodium spp.) that cause the disease depend upon ...Malaria is responsible for half a million deaths annually and poses a huge economic burden on the developing world. The mosquito-borne parasites (Plasmodium spp.) that cause the disease depend upon an unconventional actomyosin motor for both gliding motility and host cell invasion. The motor system, often referred to as the glideosome complex, remains to be understood in molecular terms and is an attractive target for new drugs that might block the infection pathway. Here, we present the high-resolution structure of the actomyosin motor complex from Plasmodium falciparum. The complex includes the malaria parasite actin filament (PfAct1) complexed with the class XIV myosin motor (PfMyoA) and its two associated light-chains. The high-resolution core structure reveals the PfAct1:PfMyoA interface in atomic detail, while at lower-resolution, we visualize the PfMyoA light-chain binding region, including the essential light chain (PfELC) and the myosin tail interacting protein (PfMTIP). Finally, we report a bare PfAct1 filament structure at improved resolution. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10590.map.gz emd_10590.map.gz | 44.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10590-v30.xml emd-10590-v30.xml emd-10590.xml emd-10590.xml | 19.9 KB 19.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_10590.png emd_10590.png | 208.6 KB | ||
| Masks |  emd_10590_msk_1.map emd_10590_msk_1.map | 512 MB |  Mask map Mask map | |
| Others |  emd_10590_additional_1.map.gz emd_10590_additional_1.map.gz emd_10590_half_map_1.map.gz emd_10590_half_map_1.map.gz emd_10590_half_map_2.map.gz emd_10590_half_map_2.map.gz | 408 MB 409.3 MB 409.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10590 http://ftp.pdbj.org/pub/emdb/structures/EMD-10590 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10590 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10590 | HTTPS FTP |
-Related structure data
| Related structure data |  6tu7MC  6tu4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10590.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10590.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Global B-factor sharpening | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.09 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10590_msk_1.map emd_10590_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_10590_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_10590_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_10590_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : PfMyoA decorated PfAct1 filament
| Entire | Name: PfMyoA decorated PfAct1 filament |
|---|---|
| Components |
|
-Supramolecule #1: PfMyoA decorated PfAct1 filament
| Supramolecule | Name: PfMyoA decorated PfAct1 filament / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Plasmodium falciparum 3D7 (eukaryote) Plasmodium falciparum 3D7 (eukaryote) |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) / Recombinant cell: Sf21 Spodoptera frugiperda (fall armyworm) / Recombinant cell: Sf21 |
-Macromolecule #1: Myosin-A
| Macromolecule | Name: Myosin-A / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Plasmodium falciparum 3D7 (eukaryote) Plasmodium falciparum 3D7 (eukaryote) |
| Molecular weight | Theoretical: 92.675477 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: GQFAVTNEEI KTASKIVRRV (SEP)NVEAFDKSG SVFKGYQIWT DISPTIENDP NIMFVKCVVQ QGSKKEKLTV VQIDPP GTG TPYDIDPTHA WNCNSQVDPM SFGDIGLLNH TNIPCVLDFL KHRYLKNQIY TTAVPLIVAI NPYKDLGNTT NEWIRRY RD TADHTKLPPH ...String: GQFAVTNEEI KTASKIVRRV (SEP)NVEAFDKSG SVFKGYQIWT DISPTIENDP NIMFVKCVVQ QGSKKEKLTV VQIDPP GTG TPYDIDPTHA WNCNSQVDPM SFGDIGLLNH TNIPCVLDFL KHRYLKNQIY TTAVPLIVAI NPYKDLGNTT NEWIRRY RD TADHTKLPPH VFTCAREALS NLHGVNKSQT IIVSGESGAG KTEATKQIMR YFASSKSGNM DLRIQTAIMA ANPVLEAF G NAKTIRNNNS SRFGRFMQLV ISHEGGIRYG SVVAFLLEKS RIITQDDNER SYHIFYQFLK GANSTMKSKF GLKGVTEYK LLNPNSTEVS GVDDVKDFEE VIESLKNMEL SESDIEVIFS IVAGILTLGN VRLIEKQEAG LSDAAAIMDE DMGVFNKACE LMYLDPELI KREILIKVTV AGGTKIEGRW NKNDAEVLKS SLCKAMYEKL FLWIIRHLNS RIEPEGGFKT FMGMLDIFGF E VFKNNSLE QLFINITNEM LQKNFVDIVF ERESKLYKDE GISTAELKYT SNKEVINVLC EKGKSVLSYL EDQCLAPGGT DE KFVSSCA TNLKENNKFT PAKVASNKNF IIQHTIGPIQ YCAESFLLKN KDVLRGDLVE VIKDSPNPIV QQLFEGQVIE KGK IAKGSL IGSQFLNQLT SLMNLINSTE PHFIRCIKPN ENKKPLEWCE PKILIQLHAL SILEALVLRQ LGYSYRRTFE EFLY QYKFV DIAAAEDSSV ENQNKCVNIL KLSGLSESMY KIGKSMVFLK QEGAKILTKI QREKLVEWEN CVSVIEAAIL KHKYK QKVN KNIPSLLRVQ AHIRKKMVAQ |
-Macromolecule #2: Actin-1
| Macromolecule | Name: Actin-1 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Plasmodium falciparum 3D7 (eukaryote) Plasmodium falciparum 3D7 (eukaryote) |
| Molecular weight | Theoretical: 42.047676 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: GAMGEEDVQA LVVDNGSGNV KAGVAGDDAP RSVFPSIVGR PKNPGIMVGM EEKDAFVGDE AQTKRGILTL KYPIEHGIVT NWDDMEKIW HHTFYNELRA APEEHPVLLT EAPLNPKGNR ERMTQIMFES FNVPAMYVAI QAVLSLYSSG RTTGIVLDSG D GVSHTVPI ...String: GAMGEEDVQA LVVDNGSGNV KAGVAGDDAP RSVFPSIVGR PKNPGIMVGM EEKDAFVGDE AQTKRGILTL KYPIEHGIVT NWDDMEKIW HHTFYNELRA APEEHPVLLT EAPLNPKGNR ERMTQIMFES FNVPAMYVAI QAVLSLYSSG RTTGIVLDSG D GVSHTVPI YEGYALPHAI MRLDLAGRDL TEYLMKILHE RGYGFSTSAE KEIVRDIKEK LCYIALNFDE EMKTSEQSSD IE KSYELPD GNIITVGNER FRCPEALFQP SFLGKEAAGI HTTTFNSIKK CDVDIRKDLY GNIVLSGGTT MYEGIGERLT RDI TTLAPS TMKIKVVAPP ERKYSVWIGG SILSSLSTFQ QMWITKEEYD ESGPSIVHRK CF |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 4 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: Jasplakinolide
| Macromolecule | Name: Jasplakinolide / type: ligand / ID: 5 / Number of copies: 4 / Formula: 9UE |
|---|---|
| Molecular weight | Theoretical: 709.67 Da |
| Chemical component information |  ChemComp-9UE: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Average electron dose: 49.2 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: a cylinder |
|---|---|
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 28.3417 Å Applied symmetry - Helical parameters - Δ&Phi: -166.498 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.1 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0 beta) / Number images used: 239021 |
 Movie
Movie Controller
Controller













 Z
Z Y
Y X
X