[English] 日本語
 Yorodumi
Yorodumi- PDB-7jn3: Cryo-EM structure of Rous sarcoma virus cleaved synaptic complex ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7jn3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Rous sarcoma virus cleaved synaptic complex (CSC) with HIV-1 integrase strand transfer inhibitor MK-2048 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE/DNA/INHIBITOR / intasome / integrase-viral DNA complex / HYDROLASE-DNA-INHIBITOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology information Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases /  ribonuclease H / DNA integration / ribonuclease H / DNA integration /  RNA-directed DNA polymerase / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / viral genome integration into host DNA / establishment of integrated proviral latency /  RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity /  Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / viral nucleocapsid ... Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / viral nucleocapsid ... Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases /  ribonuclease H / DNA integration / ribonuclease H / DNA integration /  RNA-directed DNA polymerase / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / viral genome integration into host DNA / establishment of integrated proviral latency /  RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity /  Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / viral nucleocapsid / DNA recombination / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / viral nucleocapsid / DNA recombination /  Hydrolases; Acting on ester bonds / Hydrolases; Acting on ester bonds /  DNA-directed DNA polymerase / aspartic-type endopeptidase activity / DNA-directed DNA polymerase / aspartic-type endopeptidase activity /  DNA-directed DNA polymerase activity / symbiont entry into host cell / DNA-directed DNA polymerase activity / symbiont entry into host cell /  proteolysis / proteolysis /  DNA binding / DNA binding /  RNA binding / zinc ion binding RNA binding / zinc ion bindingSimilarity search - Function | |||||||||
| Biological species |   Rous sarcoma virus Rous sarcoma virus | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.21 Å cryo EM / Resolution: 3.21 Å | |||||||||
 Authors Authors | Pandey, K.K. / Bera, S. / Shi, K. / Aihara, H. / Grandgenett, D.P. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2021 Journal: Commun Biol / Year: 2021Title: Cryo-EM structure of the Rous sarcoma virus octameric cleaved synaptic complex intasome. Authors: Krishan K Pandey / Sibes Bera / Ke Shi / Michael J Rau / Amarachi V Oleru / James A J Fitzpatrick / Alan N Engelman / Hideki Aihara / Duane P Grandgenett /  Abstract: Despite conserved catalytic integration mechanisms, retroviral intasomes composed of integrase (IN) and viral DNA possess diverse structures with variable numbers of IN subunits. To investigate ...Despite conserved catalytic integration mechanisms, retroviral intasomes composed of integrase (IN) and viral DNA possess diverse structures with variable numbers of IN subunits. To investigate intasome assembly mechanisms, we employed the Rous sarcoma virus (RSV) IN dimer that assembles a precursor tetrameric structure in transit to the mature octameric intasome. We determined the structure of RSV octameric intasome stabilized by a HIV-1 IN strand transfer inhibitor using single particle cryo-electron microscopy. The structure revealed significant flexibility of the two non-catalytic distal IN dimers along with previously unrecognized movement of the conserved intasome core, suggesting ordered conformational transitions between intermediates that may be important to capture the target DNA. Single amino acid substitutions within the IN C-terminal domain affected intasome assembly and function in vitro and infectivity of pseudotyped RSV virions. Unexpectedly, 17 C-terminal amino acids of IN were dispensable for virus infection despite regulating the transition of the tetrameric intasome to the octameric form in vitro. We speculate that this region may regulate the binding of highly flexible distal IN dimers to the intasome core to form the octameric complex. Our studies reveal key steps in the assembly of RSV intasomes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7jn3.cif.gz 7jn3.cif.gz | 353.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7jn3.ent.gz pdb7jn3.ent.gz | 285.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7jn3.json.gz 7jn3.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jn/7jn3 https://data.pdbj.org/pub/pdb/validation_reports/jn/7jn3 ftp://data.pdbj.org/pub/pdb/validation_reports/jn/7jn3 ftp://data.pdbj.org/pub/pdb/validation_reports/jn/7jn3 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  22400MC  7ku7C  7kuiC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 8 molecules ABCDEFGH
| #1: Protein |  Mass: 30926.582 Da / Num. of mol.: 8 / Fragment: UNP residues 1281-1558 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rous sarcoma virus (strain Schmidt-Ruppin A) Rous sarcoma virus (strain Schmidt-Ruppin A)Strain: Schmidt-Ruppin A / Production host:   Escherichia coli BL21(DE3) (bacteria) / Variant (production host): pLysS Escherichia coli BL21(DE3) (bacteria) / Variant (production host): pLysSReferences: UniProt: P03354, Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases, RNA-directed DNA polymerase, DNA-directed DNA polymerase, ribonuclease H, Transferases; ...References: UniProt: P03354,  Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases, Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases,  RNA-directed DNA polymerase, RNA-directed DNA polymerase,  DNA-directed DNA polymerase, DNA-directed DNA polymerase,  ribonuclease H, ribonuclease H,  Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases, Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases,  Hydrolases; Acting on ester bonds Hydrolases; Acting on ester bonds |
|---|
-DNA chain , 2 types, 4 molecules IKJL
| #2: DNA chain | Mass: 5520.600 Da / Num. of mol.: 2 / Source method: obtained synthetically Source: (synth.)   Rous sarcoma virus (strain Schmidt-Ruppin A) Rous sarcoma virus (strain Schmidt-Ruppin A)#3: DNA chain | Mass: 4899.232 Da / Num. of mol.: 2 / Source method: obtained synthetically Source: (synth.)   Rous sarcoma virus (strain Schmidt-Ruppin A) Rous sarcoma virus (strain Schmidt-Ruppin A) |
|---|
-Non-polymers , 3 types, 8 molecules 
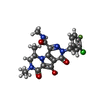



| #4: Chemical | | #5: Chemical |  MK-2048 MK-2048#6: Chemical | ChemComp-MG / |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cleaved synaptic complex (CSC) formed with Rous sarcoma virus integrase and viral DNA in presence of HIV-1 integrase strand inhibitor MK-2048 Type: COMPLEX / Entity ID: #1-#3 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.257 MDa / Experimental value: NO |
| Source (natural) | Organism:   Rous sarcoma virus (strain Schmidt-Ruppin A) Rous sarcoma virus (strain Schmidt-Ruppin A) |
| Source (recombinant) | Organism:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria) |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 0.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil |
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 105000 X / Cs Bright-field microscopy / Nominal magnification: 105000 X / Cs : 0.01 mm / Alignment procedure: BASIC : 0.01 mm / Alignment procedure: BASIC |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 66 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of real images: 5187 Details: Images were collected in movie mode at 0.2 seconds per frame. |
| EM imaging optics | Energyfilter name : GIF Bioquantum / Energyfilter slit width: 20 eV : GIF Bioquantum / Energyfilter slit width: 20 eV |
| Image scans | Movie frames/image: 40 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.18.2_3874: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
CTF correction | Type: NONE | ||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1811357 | ||||||||||||||||||||||||
3D reconstruction | Resolution: 3.21 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 456948 / Algorithm: FOURIER SPACE / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | B value: 30 / Protocol: RIGID BODY FIT / Space: REAL / Target criteria: correlation coefficient | ||||||||||||||||||||||||
| Atomic model building | PDB-ID: 5EJK Accession code: 5EJK / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller








 PDBj
PDBj










































