[English] 日本語
 Yorodumi
Yorodumi- EMDB-1499: Structure of the E. coli trigger factor bound to a translating ri... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1499 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the E. coli trigger factor bound to a translating ribosome | |||||||||
 Map data Map data | Structure of the E. coli trigger factor bound to a translating ribosome | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | trigger factor /  ribosome-nascent chain complex / translating ribosome / co-translational protein folding / ribosome-nascent chain complex / translating ribosome / co-translational protein folding /  chaperone chaperone | |||||||||
| Function / homology |  Function and homology information Function and homology information'de novo' cotranslational protein folding / stress response to copper ion / protein unfolding / chaperone-mediated protein folding / protein folding chaperone /  peptidylprolyl isomerase / peptidylprolyl isomerase /  peptidyl-prolyl cis-trans isomerase activity / peptidyl-prolyl cis-trans isomerase activity /  ribosomal large subunit assembly / large ribosomal subunit rRNA binding / ribosomal large subunit assembly / large ribosomal subunit rRNA binding /  ribosome binding ...'de novo' cotranslational protein folding / stress response to copper ion / protein unfolding / chaperone-mediated protein folding / protein folding chaperone / ribosome binding ...'de novo' cotranslational protein folding / stress response to copper ion / protein unfolding / chaperone-mediated protein folding / protein folding chaperone /  peptidylprolyl isomerase / peptidylprolyl isomerase /  peptidyl-prolyl cis-trans isomerase activity / peptidyl-prolyl cis-trans isomerase activity /  ribosomal large subunit assembly / large ribosomal subunit rRNA binding / ribosomal large subunit assembly / large ribosomal subunit rRNA binding /  ribosome binding / ribosome binding /  protein transport / cytoplasmic translation / cytosolic large ribosomal subunit / response to heat / protein transport / cytoplasmic translation / cytosolic large ribosomal subunit / response to heat /  rRNA binding / rRNA binding /  ribosome / structural constituent of ribosome / ribosome / structural constituent of ribosome /  cell cycle / cell cycle /  translation / translation /  cell division / cell division /  membrane / identical protein binding / membrane / identical protein binding /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 19.0 Å cryo EM / Resolution: 19.0 Å | |||||||||
 Authors Authors | Merz F / Boehringer D / Schaffitzel C / Preissler S / Hoffmann A / Maier T / Rutkowska A / Lozza J / Ban N / Bukau B / Deuerling E | |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2008 Journal: EMBO J / Year: 2008Title: Molecular mechanism and structure of Trigger Factor bound to the translating ribosome. Authors: Frieder Merz / Daniel Boehringer / Christiane Schaffitzel / Steffen Preissler / Anja Hoffmann / Timm Maier / Anna Rutkowska / Jasmin Lozza / Nenad Ban / Bernd Bukau / Elke Deuerling /  Abstract: Ribosome-associated chaperone Trigger Factor (TF) initiates folding of newly synthesized proteins in bacteria. Here, we pinpoint by site-specific crosslinking the sequence of molecular interactions ...Ribosome-associated chaperone Trigger Factor (TF) initiates folding of newly synthesized proteins in bacteria. Here, we pinpoint by site-specific crosslinking the sequence of molecular interactions of Escherichia coli TF and nascent chains during translation. Furthermore, we provide the first full-length structure of TF associated with ribosome-nascent chain complexes by using cryo-electron microscopy. In its active state, TF arches over the ribosomal exit tunnel accepting nascent chains in a protective void. The growing nascent chain initially follows a predefined path through the entire interior of TF in an unfolded conformation, and even after folding into a domain it remains accommodated inside the protective cavity of ribosome-bound TF. The adaptability to accept nascent chains of different length and folding states may explain how TF is able to assist co-translational folding of all kinds of nascent polypeptides during ongoing synthesis. Moreover, we suggest a model of how TF's chaperoning function can be coordinated with the co-translational processing and membrane targeting of nascent polypeptides by other ribosome-associated factors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1499.map.gz emd_1499.map.gz | 3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1499-v30.xml emd-1499-v30.xml emd-1499.xml emd-1499.xml | 10.6 KB 10.6 KB | Display Display |  EMDB header EMDB header |
| Images |  1499.gif 1499.gif | 59.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1499 http://ftp.pdbj.org/pub/emdb/structures/EMD-1499 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1499 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1499 | HTTPS FTP |
-Related structure data
| Related structure data |  2vrhMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1499.map.gz / Format: CCP4 / Size: 3.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1499.map.gz / Format: CCP4 / Size: 3.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of the E. coli trigger factor bound to a translating ribosome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.23333 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
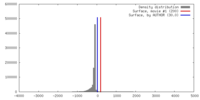
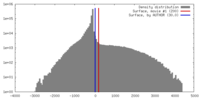
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Escherichia coli Trigger Factor associated with an Escherichia co...
| Entire | Name: Escherichia coli Trigger Factor associated with an Escherichia coli ribosome-nascent chain complex |
|---|---|
| Components |
|
-Supramolecule #1000: Escherichia coli Trigger Factor associated with an Escherichia co...
| Supramolecule | Name: Escherichia coli Trigger Factor associated with an Escherichia coli ribosome-nascent chain complex type: sample / ID: 1000 / Details: monodisperse sample / Number unique components: 2 |
|---|---|
| Molecular weight | Experimental: 2.6 MDa |
-Supramolecule #1: ribosome-nascent chain complex
| Supramolecule | Name: ribosome-nascent chain complex / type: complex / ID: 1 / Name.synonym: translating ribosome / Details: SecM-stalled E. coli ribosome complex / Recombinant expression: No / Ribosome-details: ribosome-prokaryote: ALL |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Macromolecule #1: trigger factor
| Macromolecule | Name: trigger factor / type: protein_or_peptide / ID: 1 / Name.synonym: trigger factor Details: trigger factor crosslinked to the nascent chain. Mutant TFS61C E. coli Oligomeric state: monomer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) / Strain: mutant TFS61c Escherichia coli (E. coli) / Strain: mutant TFS61c |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Details: 50 mM Hepes-KOH pH 7.5, 100 mM KCl, 25 mM MgCl2, 0.5 mg/ml chloramphenicol |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 50000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 50000 Bright-field microscopy / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Side entry liquid nitrogen-cooled cryo specimen holder Specimen holder model: GATAN LIQUID NITROGEN |
| Temperature | Average: 88 K |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 6.35 µm / Details: rotating-drum scanner |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: each image |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 19.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: IMAGIC-5, SPIDER Details: Final rounds of refinement were done using the Spider software |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Details | Protocol: Rigid Body. exhaustive search |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Cross Correlation |
| Output model |  PDB-2vrh: |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)