[English] 日本語
 Yorodumi
Yorodumi- EMDB-1484: Ribosome Binding of a Single Copy of the SecY Complex: Implicatio... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1484 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
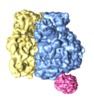
| Title | Ribosome Binding of a Single Copy of the SecY Complex: Implications for Protein Translocation | |||||||||
 Map data Map data | Escherichia coli ribosome secY complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ribosome / secY / translocation / electron microscopy | |||||||||
| Function / homology |  Function and homology information Function and homology informationintracellular protein transmembrane transport / SRP-dependent cotranslational protein targeting to membrane, translocation / signal sequence binding / stringent response / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / protein secretion / misfolded RNA binding / Group I intron splicing / protein transmembrane transporter activity ...intracellular protein transmembrane transport / SRP-dependent cotranslational protein targeting to membrane, translocation / signal sequence binding / stringent response / ornithine decarboxylase inhibitor activity / transcription antitermination factor activity, RNA binding / protein secretion / misfolded RNA binding / Group I intron splicing / protein transmembrane transporter activity / RNA folding / transcriptional attenuation / protein targeting / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / positive regulation of ribosome biogenesis / translational termination / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / assembly of large subunit precursor of preribosome / positive regulation of RNA splicing / ribosome assembly / regulation of DNA-templated transcription elongation / transcription elongation factor complex / cytosolic ribosome assembly / response to reactive oxygen species / DNA endonuclease activity / transcription antitermination / regulation of cell growth / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / protein transport / ribosome biogenesis / large ribosomal subunit / regulation of translation / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / small ribosomal subunit / 5S rRNA binding / small ribosomal subunit rRNA binding / ribosomal large subunit assembly / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.6 Å | |||||||||
 Authors Authors | Menetret JF / Schaletzky J / Clemons WM Jr / Osborne AR / Skanland SS / Denison C / Gygi SP / Kirkpatrick DS / Park E / Ludtke SJ ...Menetret JF / Schaletzky J / Clemons WM Jr / Osborne AR / Skanland SS / Denison C / Gygi SP / Kirkpatrick DS / Park E / Ludtke SJ / Rapoport TA / Akey CW | |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2007 Journal: Mol Cell / Year: 2007Title: Ribosome binding of a single copy of the SecY complex: implications for protein translocation. Authors: Jean-François Ménétret / Julia Schaletzky / William M Clemons / Andrew R Osborne / Sigrid S Skånland / Carilee Denison / Steven P Gygi / Don S Kirkpatrick / Eunyong Park / Steven J ...Authors: Jean-François Ménétret / Julia Schaletzky / William M Clemons / Andrew R Osborne / Sigrid S Skånland / Carilee Denison / Steven P Gygi / Don S Kirkpatrick / Eunyong Park / Steven J Ludtke / Tom A Rapoport / Christopher W Akey /  Abstract: The SecY complex associates with the ribosome to form a protein translocation channel in the bacterial plasma membrane. We have used cryo-electron microscopy and quantitative mass spectrometry to ...The SecY complex associates with the ribosome to form a protein translocation channel in the bacterial plasma membrane. We have used cryo-electron microscopy and quantitative mass spectrometry to show that a nontranslating E. coli ribosome binds to a single SecY complex. The crystal structure of an archaeal SecY complex was then docked into the electron density maps. In the resulting model, two cytoplasmic loops of SecY extend into the exit tunnel near proteins L23, L29, and L24. The loop between transmembrane helices 8 and 9 interacts with helices H59 and H50 in the large subunit RNA, while the 6/7 loop interacts with H7. We also show that point mutations of basic residues within either loop abolish ribosome binding. We suggest that SecY binds to this primary site on the ribosome and subsequently captures and translocates the nascent chain. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1484.map.gz emd_1484.map.gz | 10.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1484-v30.xml emd-1484-v30.xml emd-1484.xml emd-1484.xml | 10.8 KB 10.8 KB | Display Display |  EMDB header EMDB header |
| Images |  1484.png 1484.png | 666 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1484 http://ftp.pdbj.org/pub/emdb/structures/EMD-1484 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1484 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1484 | HTTPS FTP |
-Validation report
| Summary document |  emd_1484_validation.pdf.gz emd_1484_validation.pdf.gz | 325.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_1484_full_validation.pdf.gz emd_1484_full_validation.pdf.gz | 324.6 KB | Display | |
| Data in XML |  emd_1484_validation.xml.gz emd_1484_validation.xml.gz | 5.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1484 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1484 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1484 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1484 | HTTPS FTP |
-Related structure data
| Related structure data |  3bo0MC  3bo1MC  4v7iM M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1484.map.gz / Format: CCP4 / Size: 11.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1484.map.gz / Format: CCP4 / Size: 11.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Escherichia coli ribosome secY complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.73 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
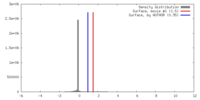
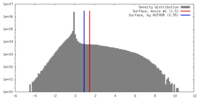
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Ribosome SecY complex
| Entire | Name: Ribosome SecY complex |
|---|---|
| Components |
|
-Supramolecule #1000: Ribosome SecY complex
| Supramolecule | Name: Ribosome SecY complex / type: sample / ID: 1000 / Oligomeric state: monomer / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 3.2 MDa |
-Supramolecule #1: 70S ribosome
| Supramolecule | Name: 70S ribosome / type: complex / ID: 1 / Name.synonym: ribosome / Recombinant expression: No / Ribosome-details: ribosome-prokaryote: ALL |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 3.2 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 50mM Hepes-KOH, 100mM KOAc, 10mM Mg(OAc)2, 0.05% DDM |
| Grid | Details: 400 mesh Cu grids with continuous carbon film |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 90 K / Instrument: HOMEMADE PLUNGER / Details: Vitrification instrument: home-made / Method: 1 second blot |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 20 |
|---|---|
| Temperature | Average: 90 K |
| Details | About 30 percent of the data were collected at 30 degree tilt and micrographs were processed in small strips parallel to the tilt axis to correct for the defocus ramp. |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 4.5 µm / Number real images: 351 / Average electron dose: 20 e/Å2 / Details: Creoscitex Eversmart scanner was used / Od range: 1 / Bits/pixel: 16 |
| Tilt angle min | 0 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 51000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: eucentric / Specimen holder model: GATAN LIQUID NITROGEN / Tilt angle max: 30 |
- Image processing
Image processing
| Details | 1900 classes were used in EMAN 3D refinement |
|---|---|
| CTF correction | Details: by micrograph |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 9.6 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: EMAN / Number images used: 39000 |
-Atomic model buiding 1
| Initial model | (PDB ID: ,  2i2t  2i2p |
|---|---|
| Software | Name:  coot coot |
| Details | Protocol: manual in O and Chimera. Loop geometry was regularized in Coot. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-3bo0:  PDB-3bo1:  PDB-4v7i: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)