+Search query
-Structure paper
| Title | Cryo-EM structures of LHCII in photo-active and photo-protecting states reveal allosteric regulation of light harvesting and excess energy dissipation. |
|---|---|
| Journal, issue, pages | Nat Plants, Vol. 9, Issue 9, Page 1547-1557, Year 2023 |
| Publish date | Aug 31, 2023 |
 Authors Authors | Meixia Ruan / Hao Li / Ying Zhang / Ruoqi Zhao / Jun Zhang / Yingjie Wang / Jiali Gao / Zhuan Wang / Yumei Wang / Dapeng Sun / Wei Ding / Yuxiang Weng /   |
| PubMed Abstract | The major light-harvesting complex of photosystem II (LHCII) has a dual regulatory function in a process called non-photochemical quenching to avoid the formation of reactive oxygen. LHCII undergoes ...The major light-harvesting complex of photosystem II (LHCII) has a dual regulatory function in a process called non-photochemical quenching to avoid the formation of reactive oxygen. LHCII undergoes reversible conformation transitions to switch between a light-harvesting state for excited-state energy transfer and an energy-quenching state for dissipating excess energy under full sunshine. Here we report cryo-electron microscopy structures of LHCII in membrane nanodiscs, which mimic in vivo LHCII, and in detergent solution at pH 7.8 and 5.4, respectively. We found that, under low pH conditions, the salt bridges at the lumenal side of LHCII are broken, accompanied by the formation of two local α-helices on the lumen side. The formation of α-helices in turn triggers allosterically global protein conformational change, resulting in a smaller crossing angle between transmembrane helices. The fluorescence decay rates corresponding to different conformational states follow the Dexter energy transfer mechanism with a characteristic transition distance of 5.6 Å between Lut1 and Chl612. The experimental observations are consistent with the computed electronic coupling strengths using multistate density function theory. |
 External links External links |  Nat Plants / Nat Plants /  PubMed:37653340 PubMed:37653340 |
| Methods | EM (single particle) |
| Resolution | 2.52 - 2.8 Å |
| Structure data | EMDB-35782, PDB-8iwx: EMDB-35783, PDB-8iwy: EMDB-35784, PDB-8iwz: EMDB-35785, PDB-8ix0: EMDB-35786, PDB-8ix1: EMDB-35787, PDB-8ix2: |
| Chemicals |  ChemComp-CHL:  ChemComp-CLA:  ChemComp-LUT:  ChemComp-XAT: 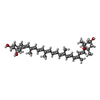 ChemComp-NEX:  ChemComp-LHG: |
| Source |
|
 Keywords Keywords |  PLANT PROTEIN / LHCII / PLANT PROTEIN / LHCII /  Light-harvesting complex / Light-harvesting complex /  Photosystem II / Photosystem II /  Photosynthesis II / Photosynthesis II /  PHOTOSYNTHESIS PHOTOSYNTHESIS |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers