+Search query
-Structure paper
| Title | Structure-based discovery of small molecules that disaggregate Alzheimer's disease tissue derived tau fibrils in vitro. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 13, Issue 1, Page 5451, Year 2022 |
| Publish date | Sep 16, 2022 |
 Authors Authors | Paul M Seidler / Kevin A Murray / David R Boyer / Peng Ge / Michael R Sawaya / Carolyn J Hu / Xinyi Cheng / Romany Abskharon / Hope Pan / Michael A DeTure / Christopher K Williams / Dennis W Dickson / Harry V Vinters / David S Eisenberg /  |
| PubMed Abstract | Alzheimer's disease (AD) is the consequence of neuronal death and brain atrophy associated with the aggregation of protein tau into fibrils. Thus disaggregation of tau fibrils could be a therapeutic ...Alzheimer's disease (AD) is the consequence of neuronal death and brain atrophy associated with the aggregation of protein tau into fibrils. Thus disaggregation of tau fibrils could be a therapeutic approach to AD. The small molecule EGCG, abundant in green tea, has long been known to disaggregate tau and other amyloid fibrils, but EGCG has poor drug-like properties, failing to fully penetrate the brain. Here we have cryogenically trapped an intermediate of brain-extracted tau fibrils on the kinetic pathway to EGCG-induced disaggregation and have determined its cryoEM structure. The structure reveals that EGCG molecules stack in polar clefts between the paired helical protofilaments that pathologically define AD. Treating the EGCG binding position as a pharmacophore, we computationally screened thousands of drug-like compounds for compatibility for the pharmacophore, discovering several that experimentally disaggregate brain-derived tau fibrils in vitro. This work suggests the potential of structure-based, small-molecule drug discovery for amyloid diseases. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:36114178 / PubMed:36114178 /  PubMed Central PubMed Central |
| Methods | EM (helical sym.) |
| Resolution | 3.3 - 3.8 Å |
| Structure data | EMDB-26663, PDB-7upe: EMDB-26664, PDB-7upf: EMDB-26665, PDB-7upg: |
| Chemicals | 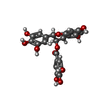 ChemComp-KDH: |
| Source |
|
 Keywords Keywords | PROTEIN FIBRIL /  Amyloid / Amyloid /  fibril / fibril /  Alzheimer's / Alzheimer's /  disease / disaggregant disease / disaggregant |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers