+Search query
-Structure paper
| Title | Structural basis for the assembly and quinone transport mechanisms of the dimeric photosynthetic RC-LH1 supercomplex. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 13, Issue 1, Page 1977, Year 2022 |
| Publish date | Apr 13, 2022 |
 Authors Authors | Peng Cao / Laura Bracun / Atsushi Yamagata / Bern M Christianson / Tatsuki Negami / Baohua Zou / Tohru Terada / Daniel P Canniffe / Mikako Shirouzu / Mei Li / Lu-Ning Liu /    |
| PubMed Abstract | The reaction center (RC) and light-harvesting complex 1 (LH1) form a RC-LH1 core supercomplex that is vital for the primary reactions of photosynthesis in purple phototrophic bacteria. Some species ...The reaction center (RC) and light-harvesting complex 1 (LH1) form a RC-LH1 core supercomplex that is vital for the primary reactions of photosynthesis in purple phototrophic bacteria. Some species possess the dimeric RC-LH1 complex with a transmembrane polypeptide PufX, representing the largest photosynthetic complex in anoxygenic phototrophs. However, the details of the architecture and assembly mechanism of the RC-LH1 dimer are unclear. Here we report seven cryo-electron microscopy (cryo-EM) structures of RC-LH1 supercomplexes from Rhodobacter sphaeroides. Our structures reveal that two PufX polypeptides are positioned in the center of the S-shaped RC-LH1 dimer, interlocking association between the components and mediating RC-LH1 dimerization. Moreover, we identify another transmembrane peptide, designated PufY, which is located between the RC and LH1 subunits near the LH1 opening. PufY binds a quinone molecule and prevents LH1 subunits from completely encircling the RC, creating a channel for quinone/quinol exchange. Genetic mutagenesis, cryo-EM structures, and computational simulations provide a mechanistic understanding of the assembly and electron transport pathways of the RC-LH1 dimer and elucidate the roles of individual components in ensuring the structural and functional integrity of the photosynthetic supercomplex. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:35418573 / PubMed:35418573 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.74 - 4.2 Å |
| Structure data | EMDB-31835, PDB-7va9: EMDB-31875, PDB-7vb9: EMDB-32042, PDB-7vnm: EMDB-32047, PDB-7vny: EMDB-32058, PDB-7vor: EMDB-32059, PDB-7vot: EMDB-32062, PDB-7voy: |
| Chemicals | 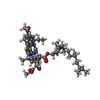 ChemComp-BCL: 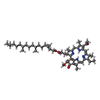 ChemComp-BPB:  ChemComp-U10:  ChemComp-PC1:  ChemComp-FE2:  ChemComp-SPO:  ChemComp-CDL:  ChemComp-BPH: 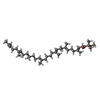 ChemComp-SPN: |
| Source |
|
 Keywords Keywords |  PHOTOSYNTHESIS / PHOTOSYNTHESIS /  photosystem / dimer / PufY-KO / photosystem / dimer / PufY-KO /  mutant / mutant /  Complex / Complex /  SUPERCOMPLEX / SUPERCOMPLEX /  purple bacteria purple bacteria |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers

















 rhodobacter sphaeroides 2.4.1 (bacteria)
rhodobacter sphaeroides 2.4.1 (bacteria)