+Search query
-Structure paper
| Title | Architecture and nucleic acids recognition mechanism of the THO complex, an mRNP assembly factor. |
|---|---|
| Journal, issue, pages | EMBO J, Vol. 31, Issue 6, Page 1605-1616, Year 2012 |
| Publish date | Mar 21, 2012 |
 Authors Authors | Alvaro Peña / Kamil Gewartowski / Seweryn Mroczek / Jorge Cuéllar / Aleksandra Szykowska / Andrzej Prokop / Mariusz Czarnocki-Cieciura / Jan Piwowarski / Cristina Tous / Andrés Aguilera / José L Carrascosa / José María Valpuesta / Andrzej Dziembowski /  |
| PubMed Abstract | The THO complex is a key factor in co-transcriptional formation of export-competent messenger ribonucleoprotein particles, yet its structure and mechanism of chromatin recruitment remain unknown. In ...The THO complex is a key factor in co-transcriptional formation of export-competent messenger ribonucleoprotein particles, yet its structure and mechanism of chromatin recruitment remain unknown. In yeast, this complex has been described as a heterotetramer (Tho2, Hpr1, Mft1, and Thp2) that interacts with Tex1 and mRNA export factors Sub2 and Yra1 to form the TRanscription EXport (TREX) complex. In this study, we purified yeast THO and found Tex1 to be part of its core. We determined the three-dimensional structures of five-subunit THO complex by electron microscopy and located the positions of Tex1, Hpr1, and Tho2 C-terminus using various labelling techniques. In the case of Tex1, a β-propeller protein, we have generated an atomic model which docks into the corresponding part of the THO complex envelope. Furthermore, we show that THO directly interacts with nucleic acids through the unfolded C-terminal region of Tho2, whose removal reduces THO recruitment to active chromatin leading to mRNA biogenesis defects. In summary, this study describes the THO architecture, the structural basis for its chromatin targeting, and highlights the importance of unfolded regions of eukaryotic proteins. |
 External links External links |  EMBO J / EMBO J /  PubMed:22314234 / PubMed:22314234 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 17.0 Å |
| Structure data | 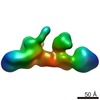 EMDB-2053: |
| Source |
|
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers




 Saccharomyces cerevisiae (brewer's yeast)
Saccharomyces cerevisiae (brewer's yeast)