+Search query
-Structure paper
| Title | Positive selection CRISPR screens reveal a druggable pocket in an oligosaccharyltransferase required for inflammatory signaling to NF-κB. |
|---|---|
| Journal, issue, pages | Cell, Vol. 187, Issue 9, Page 2209-2223.e16, Year 2024 |
| Publish date | Apr 25, 2024 |
 Authors Authors | Benjamin L Lampson / Ana S Ramίrez / Marta Baro / Lixia He / Mudra Hegde / Vidyasagar Koduri / Jamie L Pfaff / Ruth E Hanna / Julia Kowal / Nitin H Shirole / Yanfeng He / John G Doench / Joseph N Contessa / Kaspar P Locher / William G Kaelin /   |
| PubMed Abstract | Nuclear factor κB (NF-κB) plays roles in various diseases. Many inflammatory signals, such as circulating lipopolysaccharides (LPSs), activate NF-κB via specific receptors. Using whole-genome ...Nuclear factor κB (NF-κB) plays roles in various diseases. Many inflammatory signals, such as circulating lipopolysaccharides (LPSs), activate NF-κB via specific receptors. Using whole-genome CRISPR-Cas9 screens of LPS-treated cells that express an NF-κB-driven suicide gene, we discovered that the LPS receptor Toll-like receptor 4 (TLR4) is specifically dependent on the oligosaccharyltransferase complex OST-A for N-glycosylation and cell-surface localization. The tool compound NGI-1 inhibits OST complexes in vivo, but the underlying molecular mechanism remained unknown. We did a CRISPR base-editor screen for NGI-1-resistant variants of STT3A, the catalytic subunit of OST-A. These variants, in conjunction with cryoelectron microscopy studies, revealed that NGI-1 binds the catalytic site of STT3A, where it traps a molecule of the donor substrate dolichyl-PP-GlcNAc-Man-Glc, suggesting an uncompetitive inhibition mechanism. Our results provide a rationale for and an initial step toward the development of STT3A-specific inhibitors and illustrate the power of contemporaneous base-editor and structural studies to define drug mechanism of action. |
 External links External links |  Cell / Cell /  PubMed:38670073 / PubMed:38670073 /  PubMed Central PubMed Central |
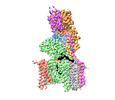
| Methods | EM (single particle) |
| Resolution | 3.61 Å |
| Structure data | EMDB-17779, PDB-8pn9: |
| Chemicals | 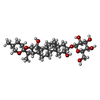 ChemComp-KZB: 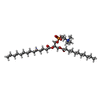 ChemComp-EGY:  ChemComp-MN: 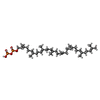 ChemComp-OTP: 
ChemComp-ZXT:  ChemComp-HOH: |
| Source |
|
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  N-glycosylation / OST-A complex / NGI-1 inhibitor N-glycosylation / OST-A complex / NGI-1 inhibitor |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers