+Search query
-Structure paper
| Title | Modulatory mechanisms of TARP γ8-selective AMPA receptor therapeutics. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 14, Issue 1, Page 1659, Year 2023 |
| Publish date | Mar 25, 2023 |
 Authors Authors | Danyang Zhang / Remigijus Lape / Saher A Shaikh / Bianka K Kohegyi / Jake F Watson / Ondrej Cais / Terunaga Nakagawa / Ingo H Greger /    |
| PubMed Abstract | AMPA glutamate receptors (AMPARs) mediate excitatory neurotransmission throughout the brain. Their signalling is uniquely diversified by brain region-specific auxiliary subunits, providing an ...AMPA glutamate receptors (AMPARs) mediate excitatory neurotransmission throughout the brain. Their signalling is uniquely diversified by brain region-specific auxiliary subunits, providing an opportunity for the development of selective therapeutics. AMPARs associated with TARP γ8 are enriched in the hippocampus, and are targets of emerging anti-epileptic drugs. To understand their therapeutic activity, we determined cryo-EM structures of the GluA1/2-γ8 receptor associated with three potent, chemically diverse ligands. We find that despite sharing a lipid-exposed and water-accessible binding pocket, drug action is differentially affected by binding-site mutants. Together with patch-clamp recordings and MD simulations we also demonstrate that ligand-triggered reorganisation of the AMPAR-TARP interface contributes to modulation. Unexpectedly, one ligand (JNJ-61432059) acts bifunctionally, negatively affecting GluA1 but exerting positive modulatory action on GluA2-containing AMPARs, in a TARP stoichiometry-dependent manner. These results further illuminate the action of TARPs, demonstrate the sensitive balance between positive and negative modulatory action, and provide a mechanistic platform for development of both positive and negative selective AMPAR modulators. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:36966141 / PubMed:36966141 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.8 - 3.3 Å |
| Structure data | EMDB-15714, PDB-8ayl: EMDB-15716, PDB-8aym: EMDB-15717, PDB-8ayn: EMDB-15718, PDB-8ayo: |
| Chemicals | 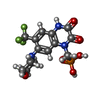 ChemComp-ZK1:  ChemComp-OLC:  ChemComp-PLM:  ChemComp-POV: 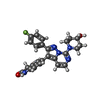 ChemComp-OIJ: 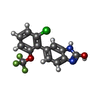 ChemComp-XVD: 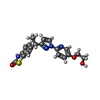 ChemComp-OLR:  ChemComp-GLU:  ChemComp-CYZ: |
| Source |
|
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  AMPAR / AMPAR /  ion channels / ion channels /  neurotransmission neurotransmission |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers












