+Search query
-Structure paper
| Title | Changes in the quaternary structure and function of MjHSP16.5 attributable to deletion of the IXI motif and introduction of the substitution, R107G, in the α-crystallin domain. |
|---|---|
| Journal, issue, pages | Philos Trans R Soc Lond B Biol Sci, Vol. 368, Issue 1617, Page 20120327, Year 2013 |
| Publish date | May 5, 2013 |
 Authors Authors | Roy A Quinlan / Yan Zhang / Andrew Lansbury / Ian Williamson / Ehmke Pohl / Fei Sun /  |
| PubMed Abstract | The archael small heat-shock protein (sHSP), MjHSP16.5, forms a 24-subunit oligomer with octahedral symmetry. Here, we demonstrate that the IXI motif present in the C-terminal domain is necessary for ...The archael small heat-shock protein (sHSP), MjHSP16.5, forms a 24-subunit oligomer with octahedral symmetry. Here, we demonstrate that the IXI motif present in the C-terminal domain is necessary for the oligomerization of MjHSP16.5. Removal increased the in vitro chaperone activity with citrate synthase as the client protein. Less predictable were the effects of the R107G substitution in MjHSP16.5 because of the differences in the oligomerization of metazoan and non-metazoan sHSPs. We present the crystal structure for MjHSP16.5 R107G and compare this with an improved (2.5 Å) crystal structure for wild-type (WT) MjHSP16.5. Although no significant structural differences were found in the crystal, using cryo-electron microscopy, we identified two 24mer species with octahedral symmetry for the WT MjHSP16.5 both at room temperature and at 60°C, all showing two major species with the same diameter of 12.4 nm. Similarly, at room temperature, there are also two kinds of 12.4 nm oligomers for R107G MjHSP16.5, but in the 60°C sample, a larger 24mer species with a diameter of 13.6 nm was observed with significant changes in the fourfold symmetry axis and dimer-dimer interface. This highly conserved arginine, therefore, contributes to the quaternary organization of non-metazoan sHSP oligomers. Potentially, the R107G substitution has functional consequences as R107G MjHSP16.5 was far superior to the WT protein in protecting βL-crystallin against heat-induced aggregation. |
 External links External links |  Philos Trans R Soc Lond B Biol Sci / Philos Trans R Soc Lond B Biol Sci /  PubMed:23530263 / PubMed:23530263 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
| Resolution | 2.85 - 15.0 Å |
| Structure data |  EMDB-2288: 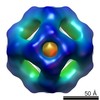 EMDB-2289: 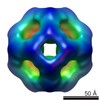 EMDB-2290:  EMDB-2291:  EMDB-2292:  EMDB-2293:  EMDB-2294:  EMDB-2295:  PDB-4i88: |
| Chemicals |  ChemComp-HOH: |
| Source |
|
 Keywords Keywords |  CHAPERONE / alpha-B domain CHAPERONE / alpha-B domain |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers





