+Search query
-Structure paper
| Title | Structural basis of kainate subtype glutamate receptor desensitization. |
|---|---|
| Journal, issue, pages | Nature, Vol. 537, Issue 7621, Page 567-571, Year 2016 |
| Publish date | Sep 22, 2016 |
 Authors Authors | Joel R Meyerson / Sagar Chittori / Alan Merk / Prashant Rao / Tae Hee Han / Mihaela Serpe / Mark L Mayer / Sriram Subramaniam /  |
| PubMed Abstract | Glutamate receptors are ligand-gated tetrameric ion channels that mediate synaptic transmission in the central nervous system. They are instrumental in vertebrate cognition and their dysfunction ...Glutamate receptors are ligand-gated tetrameric ion channels that mediate synaptic transmission in the central nervous system. They are instrumental in vertebrate cognition and their dysfunction underlies diverse diseases. In both the resting and desensitized states of AMPA and kainate receptor subtypes, the ion channels are closed, whereas the ligand-binding domains, which are physically coupled to the channels, adopt markedly different conformations. Without an atomic model for the desensitized state, it is not possible to address a central problem in receptor gating: how the resting and desensitized receptor states both display closed ion channels, although they have major differences in the quaternary structure of the ligand-binding domain. Here, by determining the structure of the kainate receptor GluK2 subtype in its desensitized state by cryo-electron microscopy (cryo-EM) at 3.8 Å resolution, we show that desensitization is characterized by the establishment of a ring-like structure in the ligand-binding domain layer of the receptor. Formation of this 'desensitization ring' is mediated by staggered helix contacts between adjacent subunits, which leads to a pseudo-four-fold symmetric arrangement of the ligand-binding domains, illustrating subtle changes in symmetry that are important for the gating mechanism. Disruption of the desensitization ring is probably the key switch that enables restoration of the receptor to its resting state, thereby completing the gating cycle. |
 External links External links |  Nature / Nature /  PubMed:27580033 / PubMed:27580033 /  PubMed Central PubMed Central |
| Methods | EM (single particle) / X-ray diffraction |
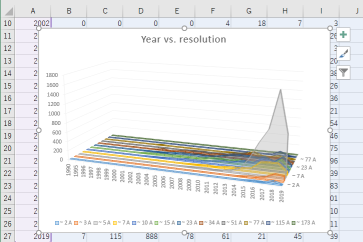
| Resolution | 1.271 - 11.6 Å |
| Structure data | EMDB-8289, PDB-5kuf:  PDB-5cmk:  PDB-5cmm: |
| Chemicals |  ChemComp-GLU:  ChemComp-LI:  ChemComp-CL: 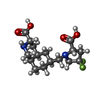 ChemComp-LY5:  ChemComp-SO4:  ChemComp-HOH: 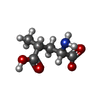 ChemComp-SYM: |
| Source |
|
 Keywords Keywords |  TRANSPORT PROTEIN / TRANSPORT PROTEIN /  Membrane protein / Membrane protein /  SIGNALING PROTEIN / GluK2EM with 2S / 4R-4-methylglutamate / GluK2EM with LY466195 SIGNALING PROTEIN / GluK2EM with 2S / 4R-4-methylglutamate / GluK2EM with LY466195 |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About EMN Papers
About EMN Papers