[English] 日本語
 Yorodumi
Yorodumi- EMDB-3523: cryoEM Structure of Polycystin-2 in complex with cations and lipids -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3523 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryoEM Structure of Polycystin-2 in complex with cations and lipids | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationdetection of nodal flow / metanephric smooth muscle tissue development / metanephric cortex development / metanephric cortical collecting duct development / metanephric distal tubule development /  polycystin complex / mesonephric tubule development / mesonephric duct development / : / metanephric part of ureteric bud development ...detection of nodal flow / metanephric smooth muscle tissue development / metanephric cortex development / metanephric cortical collecting duct development / metanephric distal tubule development / polycystin complex / mesonephric tubule development / mesonephric duct development / : / metanephric part of ureteric bud development ...detection of nodal flow / metanephric smooth muscle tissue development / metanephric cortex development / metanephric cortical collecting duct development / metanephric distal tubule development /  polycystin complex / mesonephric tubule development / mesonephric duct development / : / metanephric part of ureteric bud development / determination of liver left/right asymmetry / renal tubule morphogenesis / metanephric ascending thin limb development / HLH domain binding / basal cortex / metanephric mesenchyme development / metanephric S-shaped body morphogenesis / renal artery morphogenesis / positive regulation of inositol 1,4,5-trisphosphate-sensitive calcium-release channel activity / migrasome / cilium organization / VxPx cargo-targeting to cilium / detection of mechanical stimulus / regulation of calcium ion import / polycystin complex / mesonephric tubule development / mesonephric duct development / : / metanephric part of ureteric bud development / determination of liver left/right asymmetry / renal tubule morphogenesis / metanephric ascending thin limb development / HLH domain binding / basal cortex / metanephric mesenchyme development / metanephric S-shaped body morphogenesis / renal artery morphogenesis / positive regulation of inositol 1,4,5-trisphosphate-sensitive calcium-release channel activity / migrasome / cilium organization / VxPx cargo-targeting to cilium / detection of mechanical stimulus / regulation of calcium ion import /  cation channel complex / cation channel complex /  calcium-induced calcium release activity / muscle alpha-actinin binding / placenta blood vessel development / voltage-gated monoatomic ion channel activity / cellular response to hydrostatic pressure / outward rectifier potassium channel activity / voltage-gated monoatomic cation channel activity / non-motile cilium / cellular response to fluid shear stress / cellular response to osmotic stress / calcium-induced calcium release activity / muscle alpha-actinin binding / placenta blood vessel development / voltage-gated monoatomic ion channel activity / cellular response to hydrostatic pressure / outward rectifier potassium channel activity / voltage-gated monoatomic cation channel activity / non-motile cilium / cellular response to fluid shear stress / cellular response to osmotic stress /  voltage-gated sodium channel activity / inorganic cation transmembrane transport / voltage-gated sodium channel activity / inorganic cation transmembrane transport /  actinin binding / transcription regulator inhibitor activity / actinin binding / transcription regulator inhibitor activity /  motile cilium / determination of left/right symmetry / neural tube development / aorta development / protein heterotetramerization / ciliary membrane / branching involved in ureteric bud morphogenesis / negative regulation of G1/S transition of mitotic cell cycle / spinal cord development / heart looping / cytoplasmic side of endoplasmic reticulum membrane / motile cilium / determination of left/right symmetry / neural tube development / aorta development / protein heterotetramerization / ciliary membrane / branching involved in ureteric bud morphogenesis / negative regulation of G1/S transition of mitotic cell cycle / spinal cord development / heart looping / cytoplasmic side of endoplasmic reticulum membrane /  voltage-gated potassium channel activity / cell surface receptor signaling pathway via JAK-STAT / voltage-gated potassium channel activity / cell surface receptor signaling pathway via JAK-STAT /  potassium channel activity / centrosome duplication / sodium ion transmembrane transport / negative regulation of ryanodine-sensitive calcium-release channel activity / potassium channel activity / centrosome duplication / sodium ion transmembrane transport / negative regulation of ryanodine-sensitive calcium-release channel activity /  voltage-gated calcium channel activity / embryonic placenta development / monoatomic cation channel activity / cellular response to cAMP / release of sequestered calcium ion into cytosol / potassium ion transmembrane transport / cellular response to calcium ion / voltage-gated calcium channel activity / embryonic placenta development / monoatomic cation channel activity / cellular response to cAMP / release of sequestered calcium ion into cytosol / potassium ion transmembrane transport / cellular response to calcium ion /  cytoskeletal protein binding / basal plasma membrane / ciliary basal body / liver development / establishment of localization in cell / lumenal side of endoplasmic reticulum membrane / calcium ion transmembrane transport / protein tetramerization / cytoskeletal protein binding / basal plasma membrane / ciliary basal body / liver development / establishment of localization in cell / lumenal side of endoplasmic reticulum membrane / calcium ion transmembrane transport / protein tetramerization /  phosphoprotein binding / cytoplasmic vesicle membrane / phosphoprotein binding / cytoplasmic vesicle membrane /  cilium / intracellular calcium ion homeostasis / cilium / intracellular calcium ion homeostasis /  mitotic spindle / mitotic spindle /  Wnt signaling pathway / cellular response to reactive oxygen species / calcium ion transport / positive regulation of nitric oxide biosynthetic process / cell-cell junction / Wnt signaling pathway / cellular response to reactive oxygen species / calcium ion transport / positive regulation of nitric oxide biosynthetic process / cell-cell junction /  lamellipodium / regulation of cell population proliferation / lamellipodium / regulation of cell population proliferation /  heart development / heart development /  ATPase binding / positive regulation of cytosolic calcium ion concentration / protein homotetramerization / basolateral plasma membrane / transmembrane transporter binding / ATPase binding / positive regulation of cytosolic calcium ion concentration / protein homotetramerization / basolateral plasma membrane / transmembrane transporter binding /  regulation of cell cycle / negative regulation of cell population proliferation / regulation of cell cycle / negative regulation of cell population proliferation /  signaling receptor binding / signaling receptor binding /  calcium ion binding / endoplasmic reticulum membrane / positive regulation of gene expression / calcium ion binding / endoplasmic reticulum membrane / positive regulation of gene expression /  Golgi apparatus / Golgi apparatus /  endoplasmic reticulum / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosome endoplasmic reticulum / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosomeSimilarity search - Function | |||||||||
| Biological species |   homo sapiens (human) / homo sapiens (human) /   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.3 Å cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | Wilkes M / Madej MG / Ziegler C | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2017 Journal: Nat Struct Mol Biol / Year: 2017Title: Molecular insights into lipid-assisted Ca regulation of the TRP channel Polycystin-2. Authors: Martin Wilkes / M Gregor Madej / Lydia Kreuter / Daniel Rhinow / Veronika Heinz / Silvia De Sanctis / Sabine Ruppel / Rebecca M Richter / Friederike Joos / Marina Grieben / Ashley C W Pike / ...Authors: Martin Wilkes / M Gregor Madej / Lydia Kreuter / Daniel Rhinow / Veronika Heinz / Silvia De Sanctis / Sabine Ruppel / Rebecca M Richter / Friederike Joos / Marina Grieben / Ashley C W Pike / Juha T Huiskonen / Elisabeth P Carpenter / Werner Kühlbrandt / Ralph Witzgall / Christine Ziegler /   Abstract: Polycystin-2 (PC2), a calcium-activated cation TRP channel, is involved in diverse Ca signaling pathways. Malfunctioning Ca regulation in PC2 causes autosomal-dominant polycystic kidney disease. Here ...Polycystin-2 (PC2), a calcium-activated cation TRP channel, is involved in diverse Ca signaling pathways. Malfunctioning Ca regulation in PC2 causes autosomal-dominant polycystic kidney disease. Here we report two cryo-EM structures of distinct channel states of full-length human PC2 in complex with lipids and cations. The structures reveal conformational differences in the selectivity filter and in the large exoplasmic domain (TOP domain), which displays differing N-glycosylation. The more open structure has one cation bound below the selectivity filter (single-ion mode, PC2), whereas multiple cations are bound along the translocation pathway in the second structure (multi-ion mode, PC2). Ca binding at the entrance of the selectivity filter suggests Ca blockage in PC2, and we observed density for the Ca-sensing C-terminal EF hand in the unblocked PC2 state. The states show altered interactions of lipids with the pore loop and TOP domain, thus reflecting the functional diversity of PC2 at different locations, owing to different membrane compositions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3523.map.gz emd_3523.map.gz | 4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3523-v30.xml emd-3523-v30.xml emd-3523.xml emd-3523.xml | 12.8 KB 12.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3523.png emd_3523.png | 97.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3523 http://ftp.pdbj.org/pub/emdb/structures/EMD-3523 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3523 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3523 | HTTPS FTP |
-Related structure data
| Related structure data |  5mkeMC  3524C  5mkfC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3523.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3523.map.gz / Format: CCP4 / Size: 18.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.14 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Polycystin-2
| Entire | Name: Polycystin-2 Polycystin 2 Polycystin 2 |
|---|---|
| Components |
|
-Supramolecule #1: Polycystin-2
| Supramolecule | Name: Polycystin-2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   homo sapiens (human) homo sapiens (human) |
| Recombinant expression | Organism:   homo sapiens (human) homo sapiens (human) |
-Macromolecule #1: Polycystin-2
| Macromolecule | Name: Polycystin-2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 109.820086 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MVNSSRVQPQ QPGDAKRPPA PRAPDPGRLM AGCAAVGASL AAPGGLCEQR GLEIEMQRIR QAAARDPPAG AAASPSPPLS SCSRQAWSR DNPGFEAEEE EEEVEGEEGG MVVEMDVEWR PGSRRSAASS AVSSVGARSR GLGGYHGAGH PSGRRRRRED Q GPPCPSPV ...String: MVNSSRVQPQ QPGDAKRPPA PRAPDPGRLM AGCAAVGASL AAPGGLCEQR GLEIEMQRIR QAAARDPPAG AAASPSPPLS SCSRQAWSR DNPGFEAEEE EEEVEGEEGG MVVEMDVEWR PGSRRSAASS AVSSVGARSR GLGGYHGAGH PSGRRRRRED Q GPPCPSPV GGGDPLHRHL PLEGQPPRVA WAERLVRGLR GLWGTRLMEE SSTNREKYLK SVLRELVTYL LFLIVLCILT YG MMSSNVY YYTRMMSQLF LDTPVSKTEK TNFKTLSSME DFWKFTEGSL LDGLYWKMQP SNQTEADNRS FIFYENLLLG VPR IRQLRV RNGSCSIPQD LRDEIKECYD VYSVSSEDRA PFGPRNGTAW IYTSEKDLNG SSHWGIIATY SGAGYYLDLS RTRE ETAAQ VASLKKNVWL DRGTRATFID FSVYNANINL FCVVRLLVEF PATGGVIPSW QFQPLKLIRY VTTFDFFLAA CEIIF CFFI FYYVVEEILE IRIHKLHYFR SFWNCLDVVI VVLSVVAIGI NIYRTSNVEV LLQFLEDQNT FPNFEHLAYW QIQFNN IAA VTVFFVWIKL FKFINFNRTM SQLSTTMSRC AKDLFGFAIM FFIIFLAYAQ LAYLVFGTQV DDFSTFQECI FTQFRII LG DINFAEIEEA NRVLGPIYFT TFVFFMFFIL LNMFLAIIND TYSEVKSDLA QQKAEMELSD LIRKGYHKAL VKLKLKKN T VDDISESLRQ GGGKLNFDEL RQDLKGKGHT DAEIEAIFTK YDQDGDQELT EHEHQQMRDD LEKEREDLDL DHSSLPRPM SSRSFPRSLD DSEEDDDEDS GHSSRRRGSI SSGVSYEEFQ VLVRRVDRME HSIGSIVSKI DAVIVKLEIM ERAKLKRREV LGRLLDGVA EDERLGRDSE IHREQMERLV REELERWESD DAASQISHGL GTPVGLNGQP RPRSSRPSSS QSTEGMEGAG G NGSSNVHV |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 10 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: 4-AMINO-5-CYCLOHEXYL-3-HYDROXY-PENTANOIC ACID
| Macromolecule | Name: 4-AMINO-5-CYCLOHEXYL-3-HYDROXY-PENTANOIC ACID / type: ligand / ID: 4 / Number of copies: 8 / Formula: CHS |
|---|---|
| Molecular weight | Theoretical: 215.289 Da |
| Chemical component information | 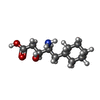 ChemComp-CHS: |
-Macromolecule #5: 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHATE
| Macromolecule | Name: 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHATE / type: ligand / ID: 5 / Number of copies: 4 / Formula: PX6 |
|---|---|
| Molecular weight | Theoretical: 647.883 Da |
| Chemical component information |  ChemComp-PX6: |
-Macromolecule #6: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 6 / Number of copies: 12 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Macromolecule #7: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 7 / Number of copies: 2 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 3200FSC |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 4.1 mm Bright-field microscopy / Cs: 4.1 mm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 1.8 e/Å2 |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
| Final reconstruction | Applied symmetry - Point group: C4 (4 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 42268 ) / Resolution.type: BY AUTHOR / Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 42268 |
-Atomic model buiding 1
| Details | We used for comparative structure modeling TRPA1 (pdb entry code 3J9P) as template for S1 and S3-S5, TRPV1 (pdb entry code 3J5Q) for S5-S6, and the TRPV2 (pdb entry code 5AN8) fitted best for S2-S3 to obtain an initial model. The soluble domain was build based on pdbID: 5K47. But we had no search model for molecular replacement. Although we had a good idea what the architecture would be like, we build the model de novo with COOT. |
|---|---|
| Output model |  PDB-5mke: |
 Movie
Movie Controller
Controller












